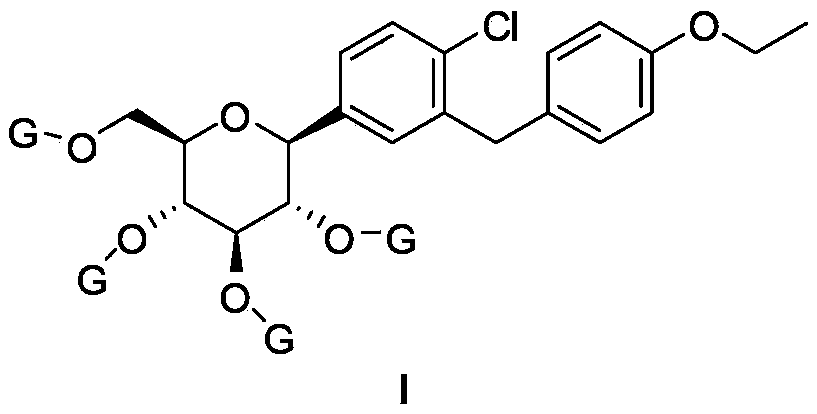
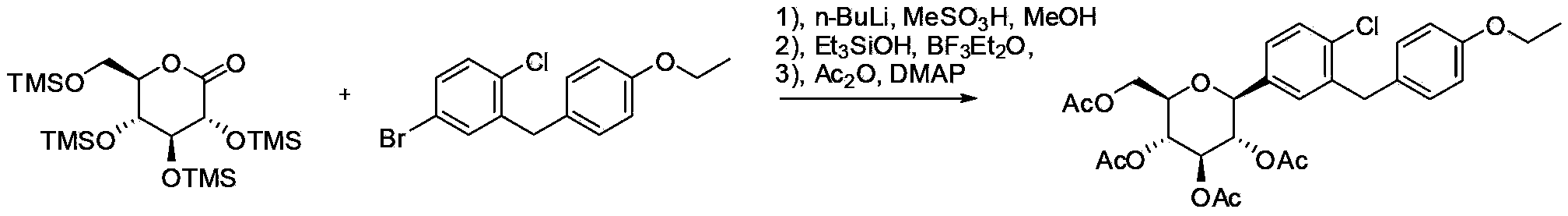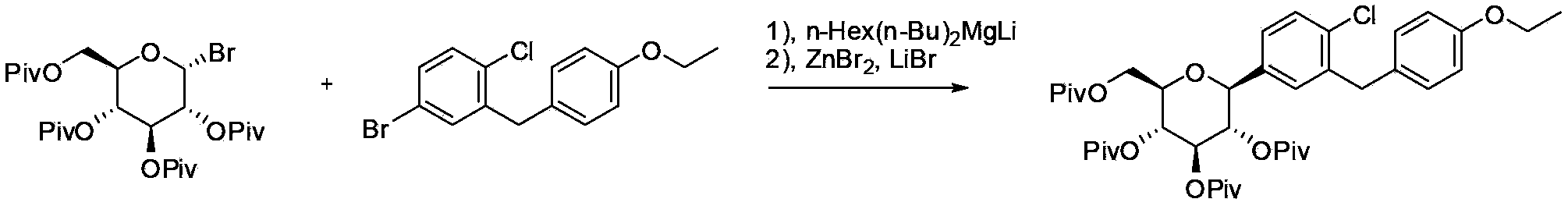Preparation method of antidiabetic dapagliflozin intermediate
A diabetes drug, dapagliflozin technology, applied in organic chemistry and other directions, can solve problems such as excessive waste and unfavorable industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1 (2R, 3R, 4R, 5S, 6S)-2-(acetylmethyl)-6-(4-chloro-3-(4-ethoxybenzyl)phenyl)tetrahydro-2H-pyran- Synthesis of 3,4,5-triacetyl ester (Ia)
[0024] Method 1): Add 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene (4.9g, 15mmol) and 20mL of tetrahydrofuran to a 50mL three-necked flask, stir and cool to -5~0°C, drop slowly Add isopropyl magnesium chloride format reagent (8mL, 2mol / L), and keep the system at 0°C and stir for 2h. In another 100mL three-necked flask, add (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-bromotetrahydro-2H-pyran-3,4,5-triacetyl ester (IIa, 4.1g, 10mmol), tetramethylethylenediamine (5wt%), cobalt triacetylacetonate (5wt%) and 20mL tetrahydrofuran, the system is cooled to 0°C. Slowly add the Grignard Reagent (IIIa) prepared in the previous 50mL bottle, after about 30min, the dripping is completed, the system is warmed to 25~30°C, the temperature is kept and stirred for 2h, the system is quenched with 1N hydrochloric acid aqueous solution, and the organic pha...
example 2
[0034] Example 2 (2S, 3S, 4R, 5R, 6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(pivaloyloxymethyl)tetrahydro-2H- Synthesis of pyran-3,4,5-tripivaloyl ester (Ib)
[0035] Add 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene (4.9g, 15mmol) and 20mL of tetrahydrofuran to a 50mL three-neck flask, stir and cool to -5~0°C, slowly add isopropyl group dropwise Magnesium chloride format reagent (8mL, 2mol / L), the system was kept at 0°C and stirred for 2h. In another 100mL three-necked flask, add (2R,3R,4S,5R,6R)-2-bromo-6-(pivaloyloxymethyl)tetrahydro-2H-pyran-3,4,5-tri Pivaloyl ester (IIb, 5.8g, 10mmol), tetramethylethylenediamine (5wt%), cobalt triacetylacetonate (5wt%) and 20mL tetrahydrofuran, the system is cooled to 0°C. Slowly add the format reagent in the previous 50mL bottle. After about 30 minutes, the addition is complete. The system is warmed to 25~30°C, kept and stirred for 2 hours. The system is quenched with 1N hydrochloric acid aqueous solution, and the organic phase is extracte...
example 3
[0037] Example 3 (2R, 3R, 4R, 5S, 6S)-3,4,5-tribenzyloxy-2-(benzyloxymethyl)-6-(4-chloro-3-(4-ethoxybenzyl) ) Phenyl) tetrahydro-2H-pyran (Ic) synthesis
[0038] Add 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene (4.9g, 15mmol) and 20mL of tetrahydrofuran to a 50mL three-neck flask, stir and cool to -5~0°C, slowly add isopropyl group dropwise Magnesium chloride format reagent (8mL, 2mol / L), the system was kept at 0°C and stirred for 2h. In another 100mL three-necked flask, add (2R,3R,4S,5R,6R)-3,4,5-tribenzyloxy-2-(benzyloxymethyl)-6-bromotetrahydro-2H-pyridine Cyan (IIc, 6.0g, 10mmol), tetramethylethylenediamine (5wt%), cobalt triacetylacetonate (5wt%) and 20mL tetrahydrofuran, the system is cooled to 0°C. Slowly add the format reagent in the previous 50mL bottle. After about 30 minutes, the addition is complete. The system is warmed to 25~30°C, kept and stirred for 2 hours. The system is quenched with 1N hydrochloric acid aqueous solution, and the organic phase is extracted with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com