A kind of preparation method of phosphoric sugar alcohol tetrahydrothiazole-4-carboxylic acid compound
A technology of carboxylic acid compound and tetrahydrothiazole, which is applied in the field of preparation of phosphate sugar alcohol tetrahydrothiazole-4-carboxylic acid compound to achieve the effect of supplementing the loss of metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
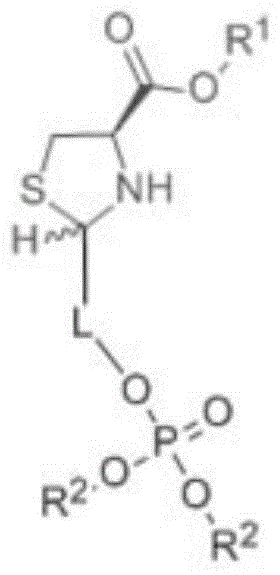
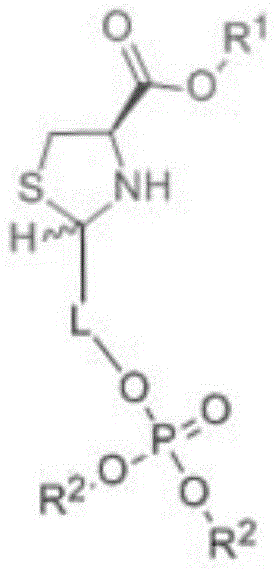
[0051] The preparation method of the first phosphate sugar alcohol tetrahydrothiazole-4-carboxylic acid compound comprises the following steps:
[0052] A kind of preparation method of phosphoric sugar alcohol tetrahydrothiazole-4-carboxylic acid compound, comprises the following steps:
[0053] 1) Selecting one of cysteine or phosphate sugar alcohol compounds as a raw material, dissolving the above compounds with a polar solvent;
[0054] 2) Choose the one with R 2 Phosphate sugar alcohol compound solution with functional groups or with R 1 Cysteine solution of functional groups, stirred at room temperature for 12-24 hours; here R 2 Phosphate sugar alcohol compound solution with functional groups or with R 1 The selection of the cysteine solution of functional group is based on: when selecting cysteine as raw material in step 1), what is selected in step 2) is to have R 2 The phosphate sugar alcohol compound solution of functional group; When step 1) selects phosp...
Embodiment 1
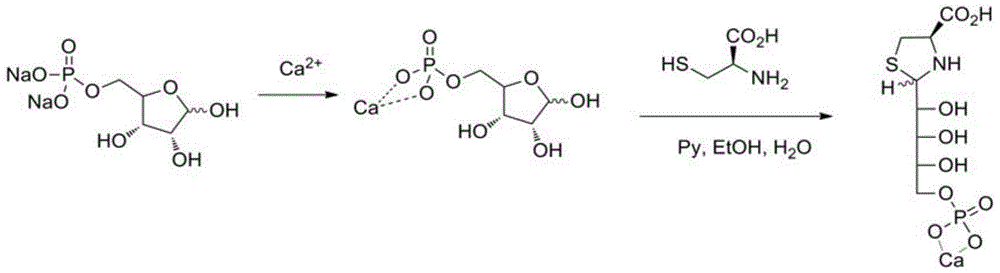
[0064] Weigh 27.4 kg of D-ribose-5-phosphate disodium salt, stir with 50 kg of deionized water until completely dissolved, add 12.2 kg of calcium chloride solid in 18 kg of water, and stir at room temperature for more than 12 hours. Add 150 kg of ethanol, stir for 2 hours, filter, collect the solid, wash with ethanol, and obtain 25.5 kg of white solid product as D-ribose-5-phosphate calcium salt. In a nitrogen-protected reaction vessel, weigh 25.5 kg of D-ribose-5-phosphate calcium salt, add 100 kg of deionized water and stir until completely dissolved, then add 15 kg of L-cysteine hydrochloride, and stir until completely Dissolve, add 7.52 kg of pyridine, and stir at room temperature for more than 24 hours. Add 300 kg of ethanol, lower the temperature to less than 5 degrees Celsius, stir for 2 hours, filter and wash with cold ethanol to obtain 30.36 kg of white solid product. The yield was 86%, and the product was confirmed by NMR, IR and mass spectrometry.
Embodiment 2
[0066] Weigh 27.4 kg of D-ribose-5-phosphate disodium salt, stir with 50 kg of deionized water until completely dissolved, add 13.94 kg of ferrous chloride dissolved in 25 kg of water, and stir at room temperature for more than 12 hours. Add 150 kg of ethanol, stir for 2 hours, filter, collect the solid, wash with ethanol, and obtain 26.9 kg of white solid product which is ferrous D-ribose-5-phosphate. In a nitrogen-protected reaction vessel, weigh 26.9 kilograms of D-ribose-5-ferrous phosphate, add 100 kilograms of deionized water and stir until completely dissolved, then add 15 kilograms of D-cysteine hydrochloride, and stir until After completely dissolving, 7.52 kg of pyridine was added and stirred at room temperature for more than 24 hours. Add 300 kg of ethanol, lower the temperature to less than 5 degrees Celsius, stir for 2 hours, filter and wash with cold ethanol to obtain 29.78 kg of white solid product. The yield was 81%, and the product was confirmed by NMR, IR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com