Hyaluronic acid modified ethosome, acupoint drug delivery system containing hyaluronic acid modified ethosome and application of system
A technology of hyaluronic acid modification and hyaluronic acid, which is applied in the field of medicine, can solve the problems of drug loss and slow diffusion, and achieve the effect of enhancing absorption, increasing delivery, and improving the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Construction of a novel acupoint drug delivery system with hyaluronic acid-modified ethosomes (HA-ES) loaded with Dingguisan volatile oil
[0059] 1.1 Extraction of Dingguisan volatile oil
[0060] Referring to the clove and cinnamon medicinal materials stipulated in the 2015 edition of "Chinese Pharmacopoeia", weigh 200.3g clove and 200.6g cinnamon medicinal materials, crush them into a 20-mesh coarse powder, add 8 times the amount of water, soak for 1h, and extract by steam distillation at 200°C for 6h , to collect the volatile oil. Get 3mL cinnamon volatile oil and 15mL clove volatile oil. After it is mixed evenly, it is used as Ding Gui scattered volatile oil. Using HPLC detection, the contents of eugenol and cinnamon aldehyde in clove volatile oil and cinnamon volatile oil were 85.8±0.43% and 93.1±4.45%, respectively, which met the quality standards for “clove” and “cinnamon” medicinal materials in the 2015 edition of the Chinese Pharmacopoeia.
[0061] 1.2 Synt...
Embodiment 2
[0066] Evaluation and characterization of hyaluronic acid-modified ethosomes (HA-ES) loaded with Dingguisan volatile oil:
[0067] 2.1 Determination of particle size and potential
[0068] The particle size distribution of each nano-preparation was measured by a dynamic light scattering method with a Malvern particle size potentiometer. Three samples were prepared in parallel, and the particle size and Zeta potential were measured. The results showed that the average particle size of HA-ES loaded with Dingguisan volatile oil was 299.5±54.3nm, and the Zeta potential was -31.7±0.47mV.
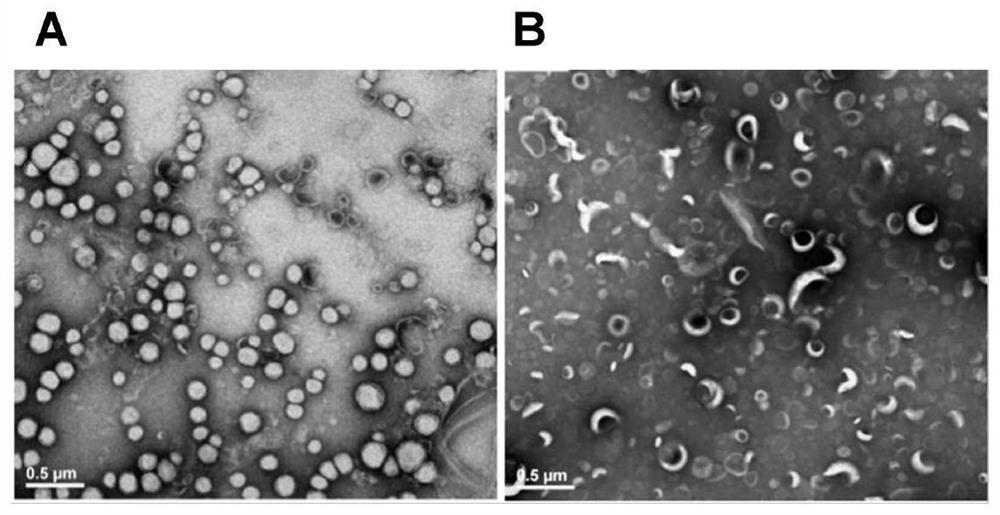
[0069] 2.2 Transmission electron microscope observation
[0070] Each preparation was diluted with deionized water to a certain multiple, dropped on the copper grid, and after natural drying, it was negatively stained with uranyl acetate for 2 minutes, and then the excess negative staining solution was absorbed with filter paper, dried, and observed under a transmission electron microscope Pre...
Embodiment 3
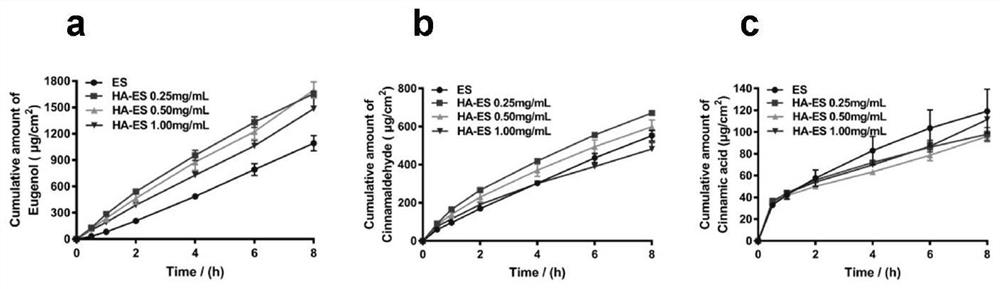
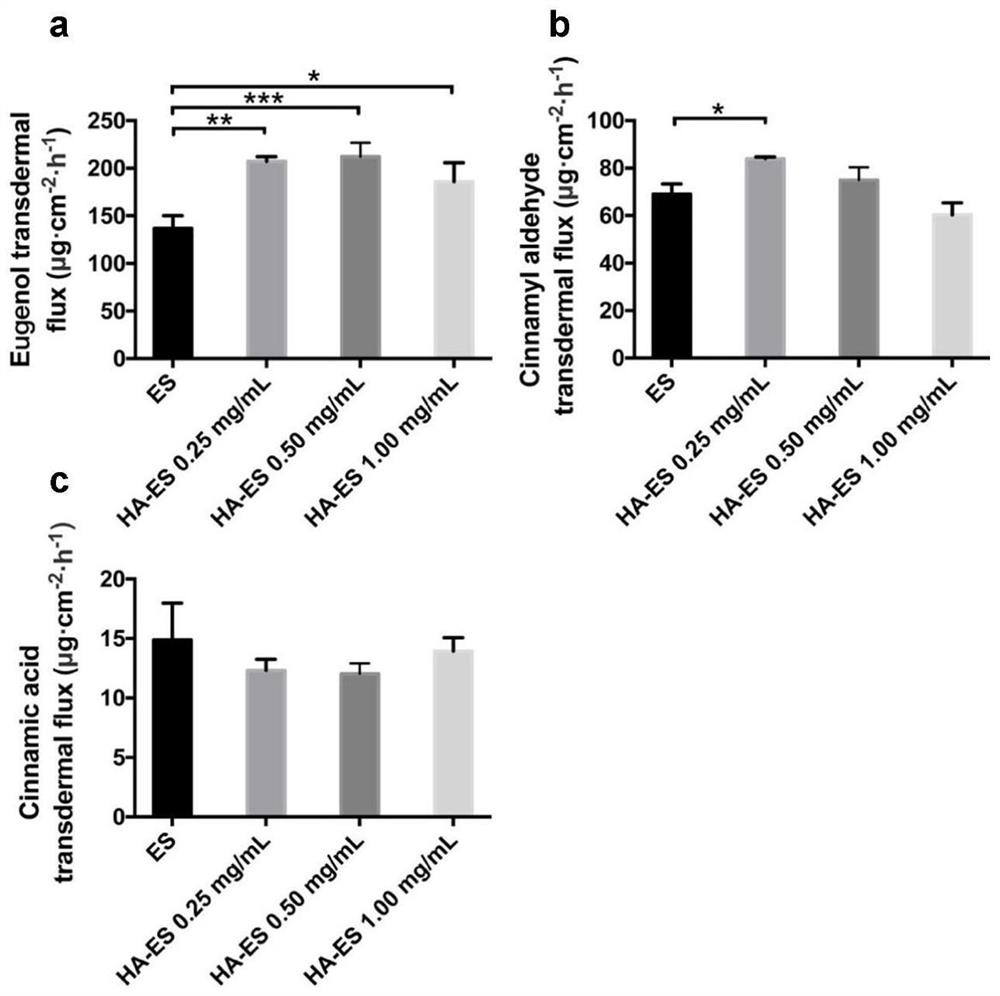
[0084] In vitro transdermal behavior:
[0085] 3.1 In vitro transdermal behavior
[0086] Take SD rats, anesthetized with 10% chloral hydrate, depilate the abdomen with a razor, scrub with normal saline, peel off the abdominal skin with a scalpel, remove subcutaneous tissue and fat, wash with normal saline, store in a -4°C refrigerator, and use within one week , Check the integrity of the mouse skin carefully before use. The modified Franze diffusion cell was used, and the isolated mouse skin was taken, with the cuticle facing the supply pool and the subcutaneous tissue facing the receiving pool. The receiving tank was filled with receiving solution (12.5 mL of 10% PEG-200 / PBS [w / v] solution), the air bubbles were exhausted, and the mixture was equilibrated for 30 min. Add 1mL of the preparation to the supply pool, so that the stratum corneum layer is in good contact with the preparation. The supply cell was sealed with a parafilm, and the receiving solution was magneticall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com