Abiraterone acetate tablet and preparing method thereof
A technology for abiraterone acetate and tablets, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as poor crystal fluidity, unfavorable crystal form stable storage, and crystal form destruction threats. , to achieve the effect of simple preparation method, excellent stability and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
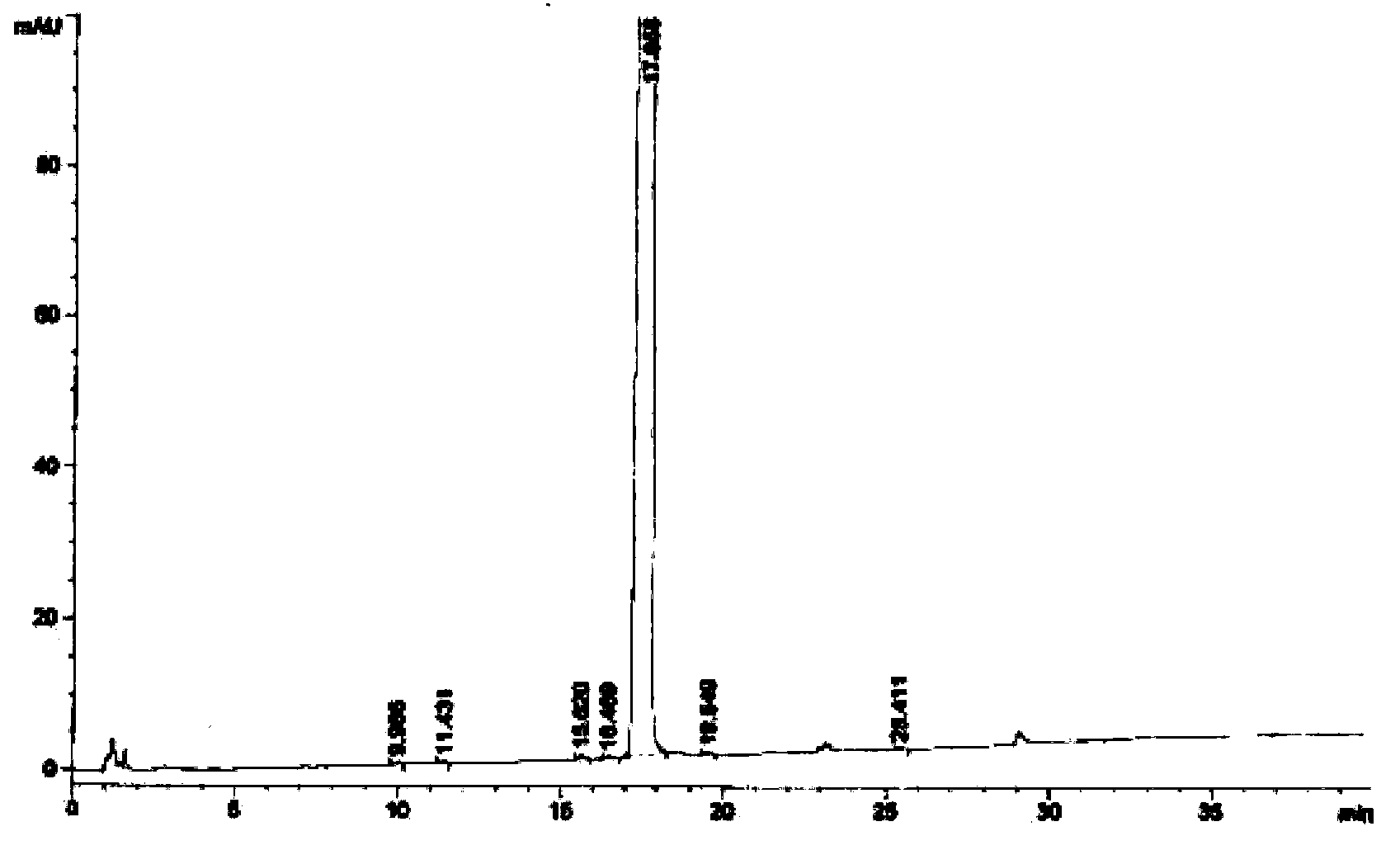
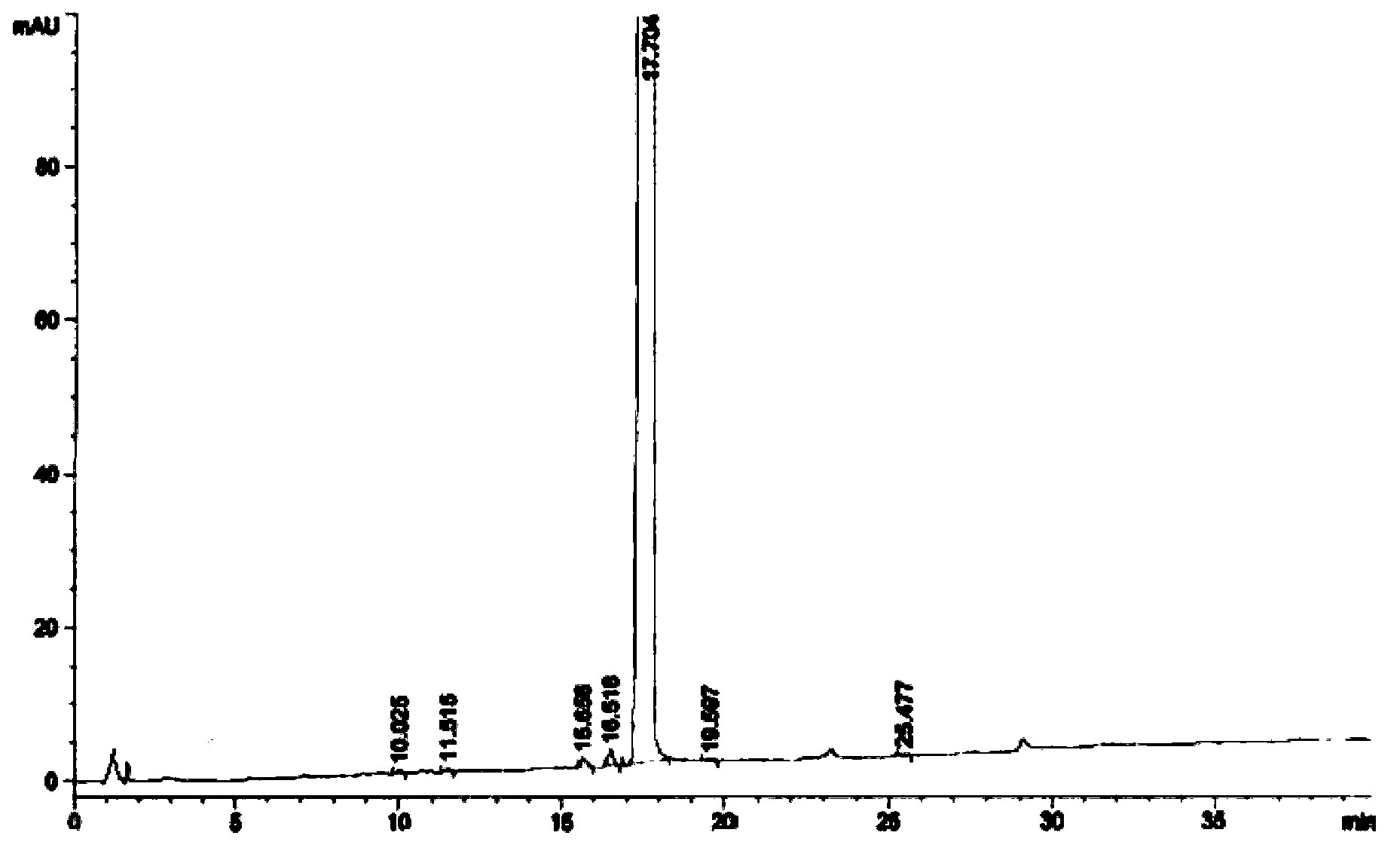
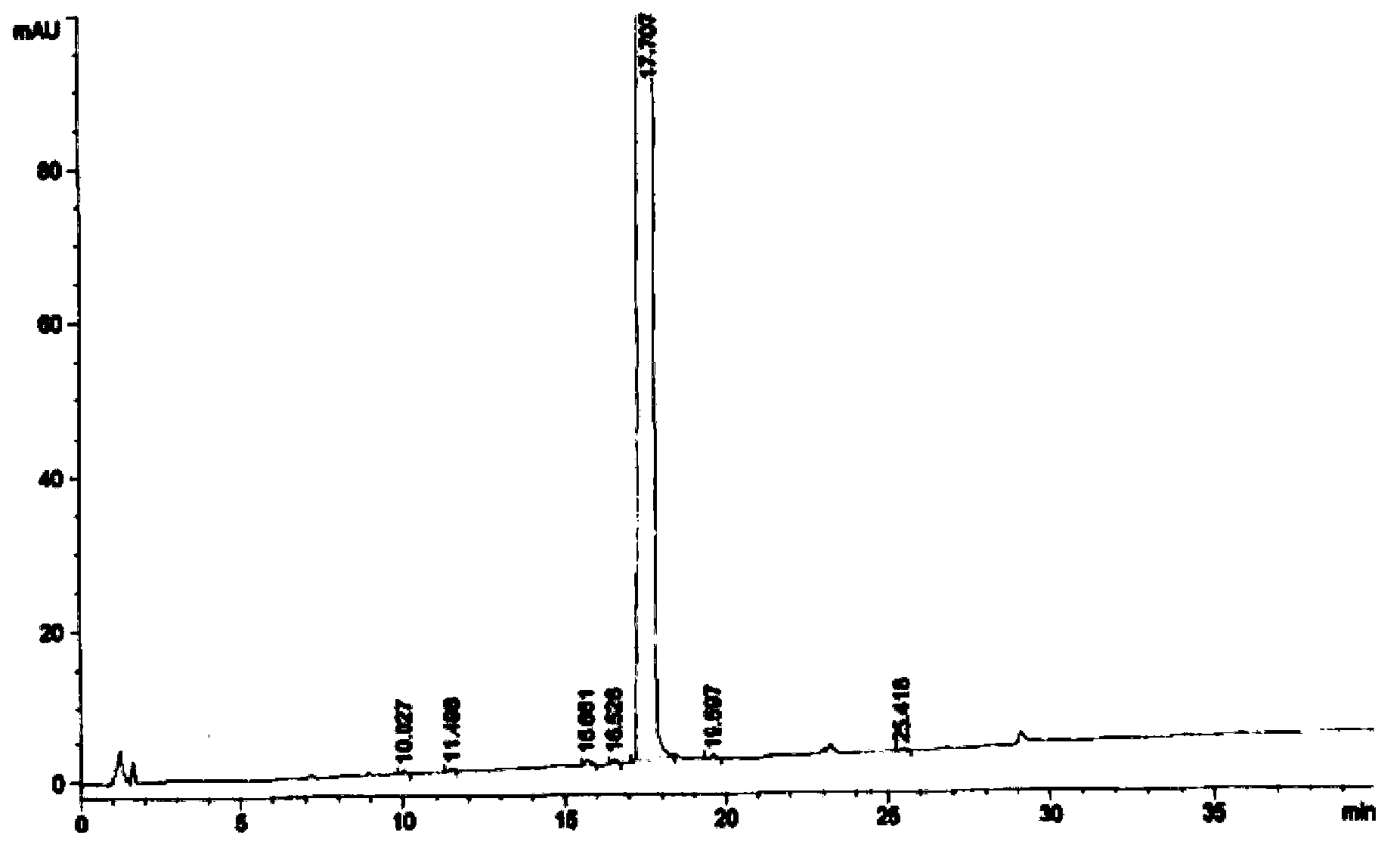
Image
Examples
Embodiment 1
[0062] Abiraterone Acetate Tablets
[0063] Component
%(w / w)
34.97
lactose monohydrate
27.82
21.21
10.00
povidone
3.50
1.00
1.50
[0064] 1. Preparation process:
[0065] 1.1 Preparation before ingredients
[0066] 1.1.1 Screening
[0067] Get the crushed abiraterone acetate and pass through a 60-mesh sieve for subsequent use.
[0068] 1.2 Ingredients
[0069] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0070] 1.3 Hybrid
[0071] Weigh the prescribed amount of abiraterone acetate, 0.5% sodium lauryl sulfate, lactose monohydrate, microcrystalline cellulose and 3% croscarmellose sodium, and mix well.
[0072] 1.4 Granulation
[0073] Add 50% ethanol solution containing 10% PVP to the above mixture to make soft material, pa...
Embodiment 2
[0080] Abiraterone Acetate Tablets
[0081] Component
%(w / w)
34.97
25.00
23.53
10.00
povidone
3.50
1.50
1.50
[0082] 1. Preparation process:
[0083] 1.1 Preparation before ingredients
[0084] 1.1.1 Screening
[0085] Get the crushed abiraterone acetate and pass through a 60-mesh sieve for subsequent use.
[0086] 1.2 Ingredients
[0087] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0088] 1.3 Hybrid
[0089] Weigh the prescribed amount of abiraterone acetate, 1.0% sodium lauryl sulfate, lactose monohydrate, microcrystalline cellulose and 3% croscarmellose sodium, and mix well.
[0090] 1.4 Granulation
[0091] Add 50% ethanol solution containing 10% PVP to the above mixture to make soft material, ...
Embodiment 3
[0098] Abiraterone Acetate Tablets
[0099] Component
%(w / w)
34.97
27.82
20.21
10.00
povidone
3.50
2.00
Magnesium stearate
1.50
[0100] 1. Preparation process:
[0101] 1.1 Preparation before ingredients
[0102] 1.1.1 Screening
[0103] Get the crushed abiraterone acetate and pass through a 60-mesh sieve for subsequent use.
[0104] 1.2 Ingredients
[0105] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0106] 1.3 Hybrid
[0107] Weigh the prescribed amount of abiraterone acetate, 1.0% sodium lauryl sulfate, lactose monohydrate, microcrystalline cellulose and 3% croscarmellose sodium, and mix well.
[0108] 1.4 Granulation
[0109] Add 50% ethanol solution containing 10% PVP to the above mixture to make soft material, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesh | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com