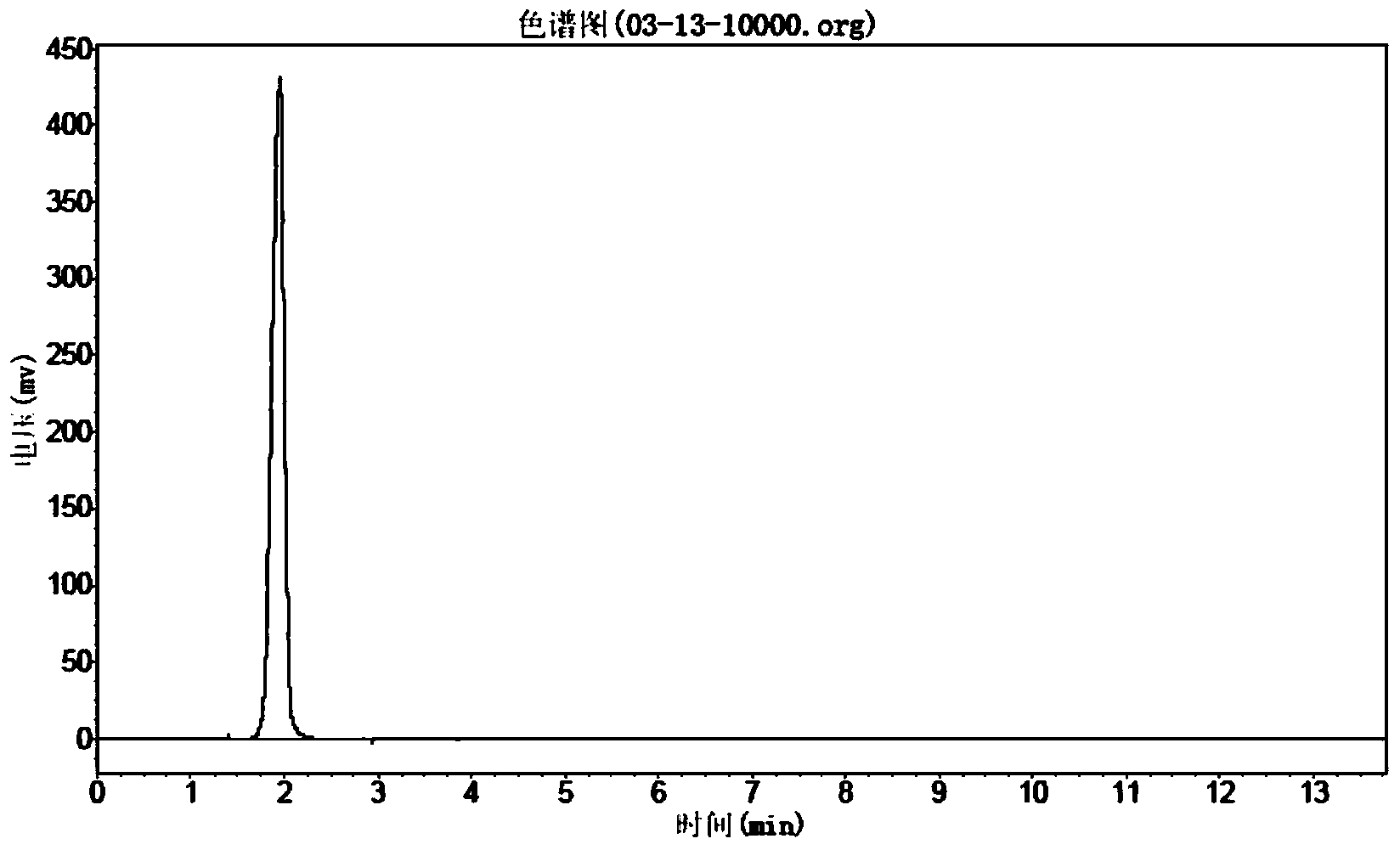
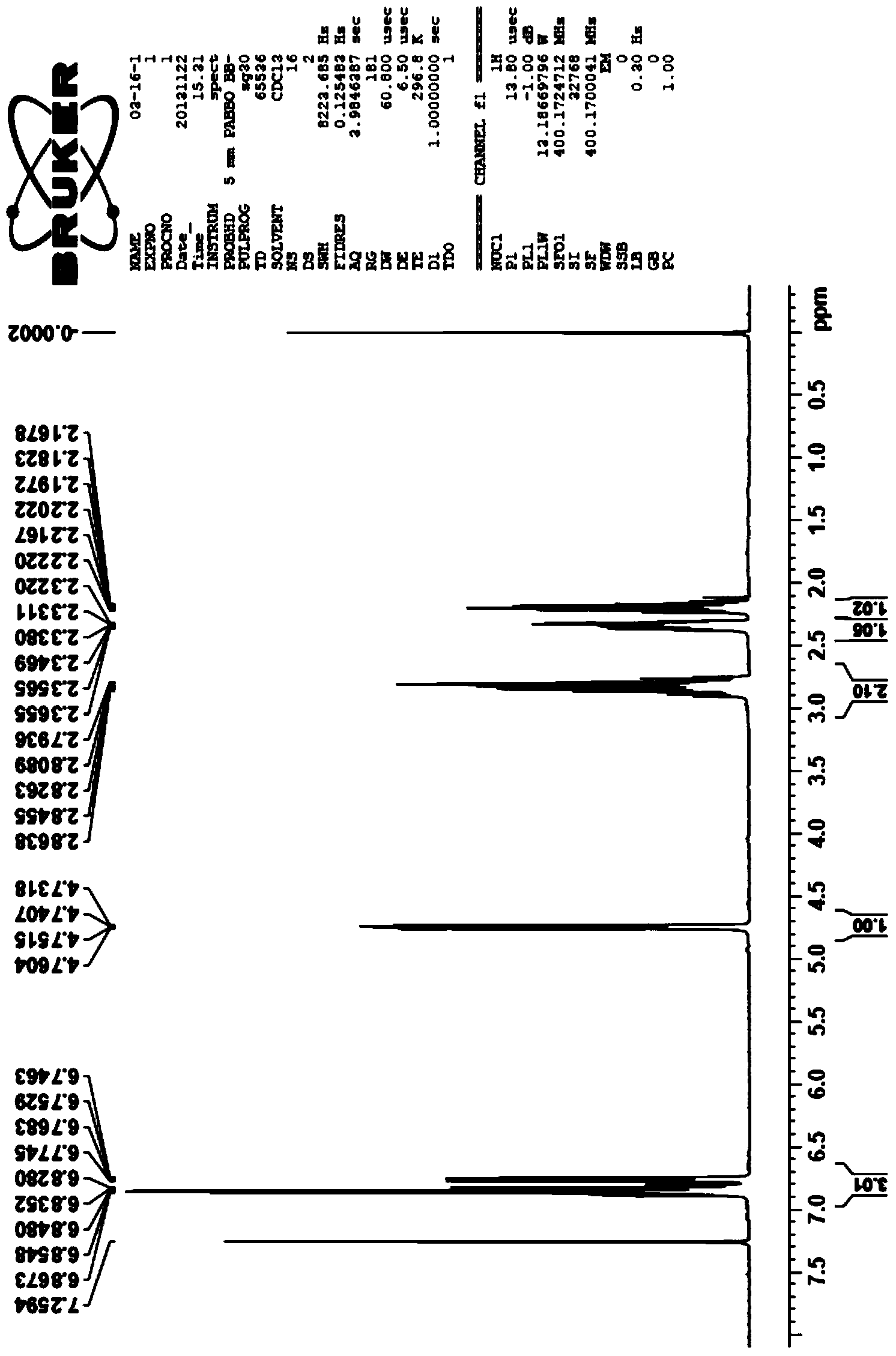
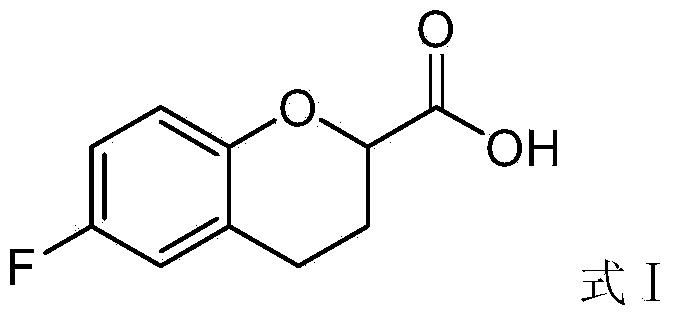
Preparation method of 6-fluorochroman-2-formic acid
A technology of formic acid and chroman, which is applied in the field of synthesis of chemical drug intermediates, can solve the problems of increased environmental protection costs, too long reaction steps, increased production costs, etc., and achieves the effect of low price, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: The preparation method of 6-fluorochroman-2-formic acid, the steps are as follows:
[0056] 1) Synthesis of 2-(p-fluorophenoxy)butenedioic acid
[0057] Add 44.8 g (0.4 mol) of p-fluorophenol into a four-necked flask, add 25 mL of methanol, and stir to dissolve. Weigh 56.8 g (0.4 mol) of butynedioic acid dimethyl ester and dissolve it in 15 mL of methanol, add it dropwise to the above-mentioned methanol solution of p-fluorophenol, stir well, there is no obvious heating phenomenon, and the solution appears light yellow. Weigh 2.0g (0.02mol) of triethylamine and slowly add it dropwise to the above reaction system, a large amount of heat will be generated. When dropping triethylamine, keep the temperature of the reaction system below 35°C, and the solution will gradually turn brown. After the addition of triethylamine is completed, keep the reaction system at 25-35°C for 1 hour. As the reaction progresses, a large amount of solids are gradually produced in t...
Embodiment 2
[0063] Embodiment 2: comparative example
[0064] The 2-(p-fluorophenoxy group) butenedioic acid dimethyl ester synthesized in the reaction process is separated, and the specific process is as follows
[0065] 1) Synthesis of 2-(p-fluorophenoxy)butenedioic acid dimethyl ester
[0066] Add 44.8g (0.4mol) of p-fluorophenol into a four-necked flask, add 25ml of methanol, and stir to dissolve. Weigh 56.8 g (0.4 mol) of dimethyl butynedate and dissolve it in 15 ml of methanol, add dropwise to the above-mentioned methanol solution of p-fluorophenol, stir well, there is no obvious temperature rise, and the solution appears light yellow. Weigh 2.0g (0.02mol) of triethylamine and slowly add it dropwise to the above reaction system, a large amount of heat will be generated. When dropping triethylamine, keep the temperature of the reaction system below 35°C, and the solution will gradually turn brown. After the triethylamine is added dropwise, keep the reaction system at 25-35°C for 1 ...
Embodiment 3
[0072] As described in Example 1, the difference is that in step 1) the concentrated hydrochloric acid used for acidification is used to prepare 6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid in the previous batch to obtain The sulfuric acid filtrate (the molar ratio of the hydrogen element in the alkali and the sulfuric acid solution is 1:1-1.5) replaces and carries out the acidification reaction, and the sulfuric acid aqueous solution obtained by filtering the extraction reaction as described in the embodiment 1 step 2 also obtains a good The effect of 2-(p-fluorophenoxy)butenedioic acid solid, the yield is 85.1%, and the purity is 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com