Nickel removing method for magnesium alloy
A magnesium alloy, nickel content technology, applied in the field of magnesium alloy nickel removal, can solve the problem of no mention of magnesium alloy nickel removal scheme, difficulty in controlling the aluminum content of magnesium alloy, reducing the scope of use of magnesium alloy, etc., to achieve expanded reuse range, short response time, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
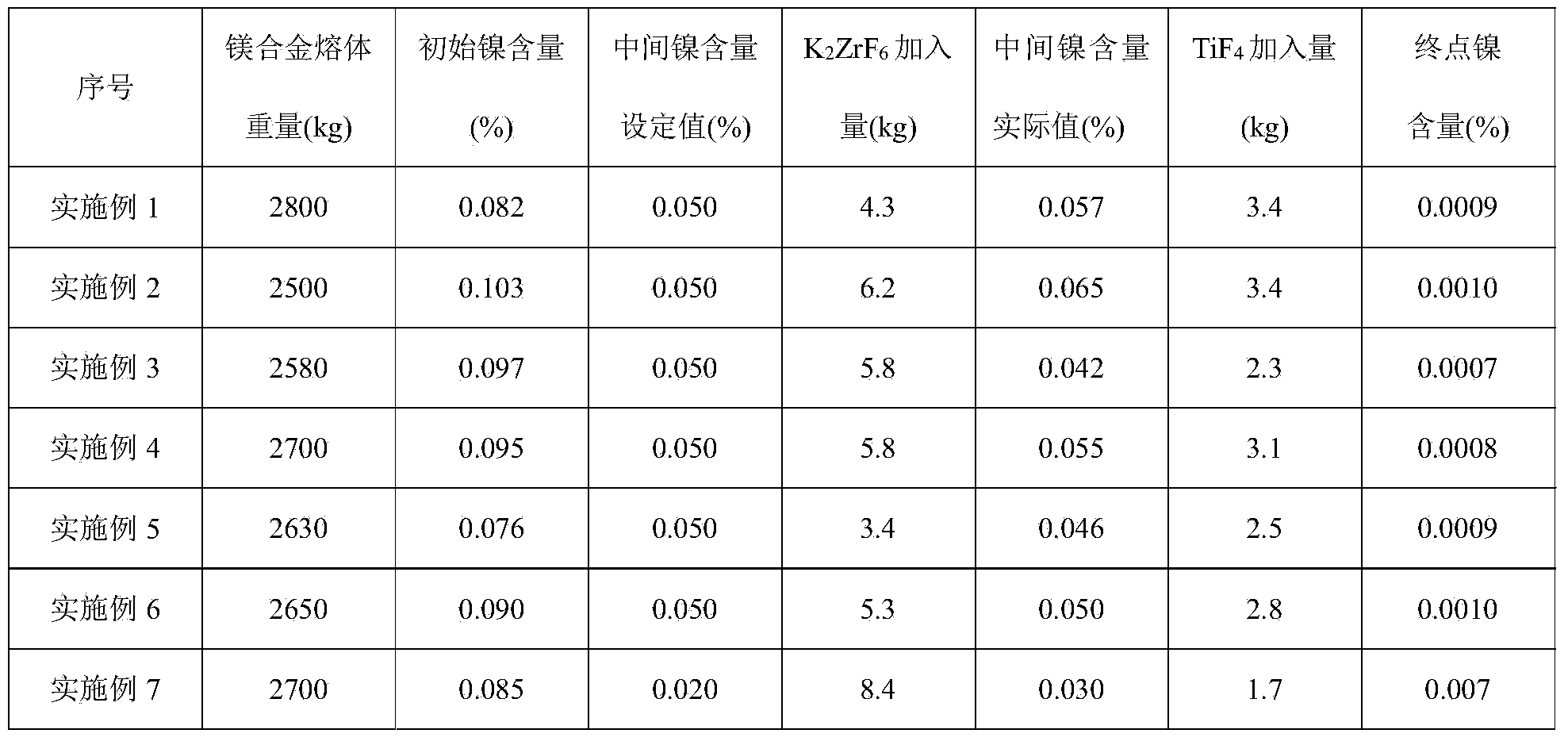
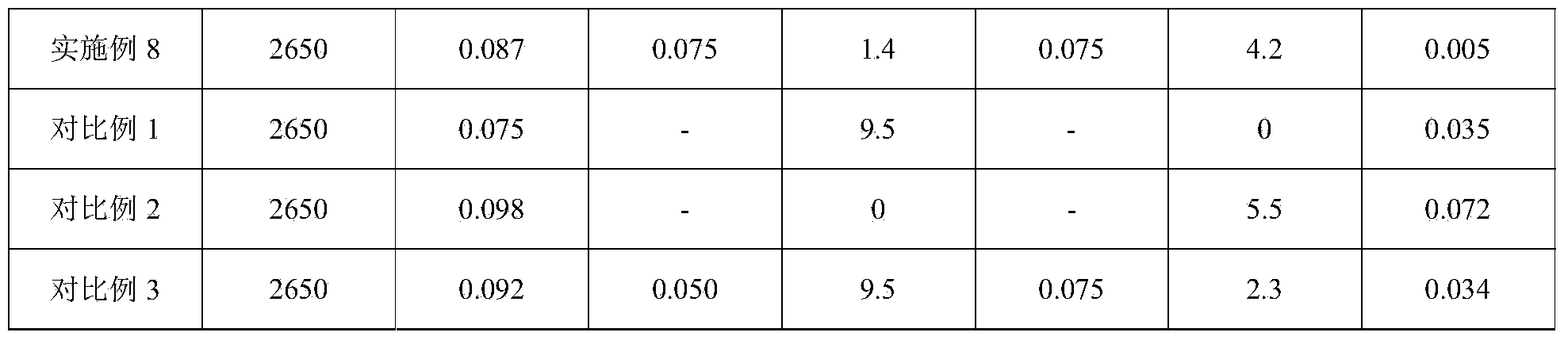
[0036] (1) carry out spectroscopic analysis to the complete magnesium alloy melt sampling of melting, Ni content is 0.082%;
[0037] (2) The weight of magnesium alloy melt is 2800kg, set the Ni content to 0.05%, weigh 4.3kg K 2 ZrF 6 ,spare;
[0038] (3) The above prepared 4.3kg K 2 ZrF 6 Mix evenly with the flux used for magnesium alloy refining;
[0039] (4) Keep the temperature of the metal melt at 725° C., add the above-mentioned uniformly mixed flux, and stir mechanically for 15 minutes;
[0040] (5) the slag that sinks into the bottom of the crucible after the above-mentioned reaction is pulled out;
[0041] (6) sampling, spectral analysis Ni content is 0.057%;
[0042] (7) Weigh 3.4kgTiF 4 ,spare;
[0043] (8) the TiF prepared above 4 Add the magnesium alloy melt during the alloying process and fully stir for 15 minutes;
[0044] (9) the above-mentioned fully reacted magnesium alloy melt was left to stand for 35 minutes;
[0045] (10) Sampling and analyzing t...
Embodiment 2
[0048] (1) carry out spectroscopic analysis to the complete magnesium alloy melt sampling of melting, Ni content is 0.103%;
[0049] (2) The weight of the magnesium alloy melt is 2500kg, set the Ni content to 0.05%, weigh 6.2kg K 2 ZrF 6 ,spare;
[0050] (3) The above prepared 6.2kg K 2 ZrF 6 Mix evenly with the flux used for refining;
[0051] (4) Keep the temperature of the metal melt at 728° C., add the above-mentioned homogeneously mixed flux, and stir mechanically for 18 minutes;
[0052] (5) the slag that sinks into the bottom of the crucible after the above-mentioned reaction is pulled out;
[0053] (6) sampling, spectral analysis Ni content is 0.065%;
[0054] (7) Weigh 3.4kgTiF 4 ,spare;
[0055] (8) the TiF prepared above 4 Add the magnesium alloy melt during the alloying process and fully stir for 18 minutes;
[0056] (9) the above-mentioned fully reacted magnesium alloy melt was left to stand for 32 minutes;
[0057] (10) Sampling and analyzing the above...
Embodiment 3
[0060] (1) carry out spectroscopic analysis to the complete magnesium alloy melt sampling of melting, Ni content is 0.097%;
[0061] (2) The weight of magnesium alloy melt is 2580kg, set the Ni content to 0.05%, weigh 5.8kg K 2 ZrF 6 ,spare;
[0062] (3) The above prepared 5.8kg K 2 ZrF 6 Mix evenly with the flux used for refining;
[0063] (4) Keep the temperature of the metal melt at 720° C., add the above-mentioned uniformly mixed flux, and stir mechanically for 20 minutes;
[0064] (5) the slag that sinks into the bottom of the crucible after the above-mentioned reaction is pulled out;
[0065] (6) sampling, spectral analysis Ni content is 0.042%;
[0066] (7) Weigh 2.3kgTiF 4 ,spare;
[0067] (8) the TiF prepared above 4 Add the magnesium alloy melt during the alloying process and fully stir for 20 minutes;
[0068] (9) the above-mentioned fully reacted magnesium alloy melt was left to stand for 30 minutes;
[0069] (10) Sampling and analyzing the above magnesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com