Method and composition for wound treatment
A technology of composition and matrix composition, which is applied in the direction of drug combination, non-active ingredients of polymer compounds, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
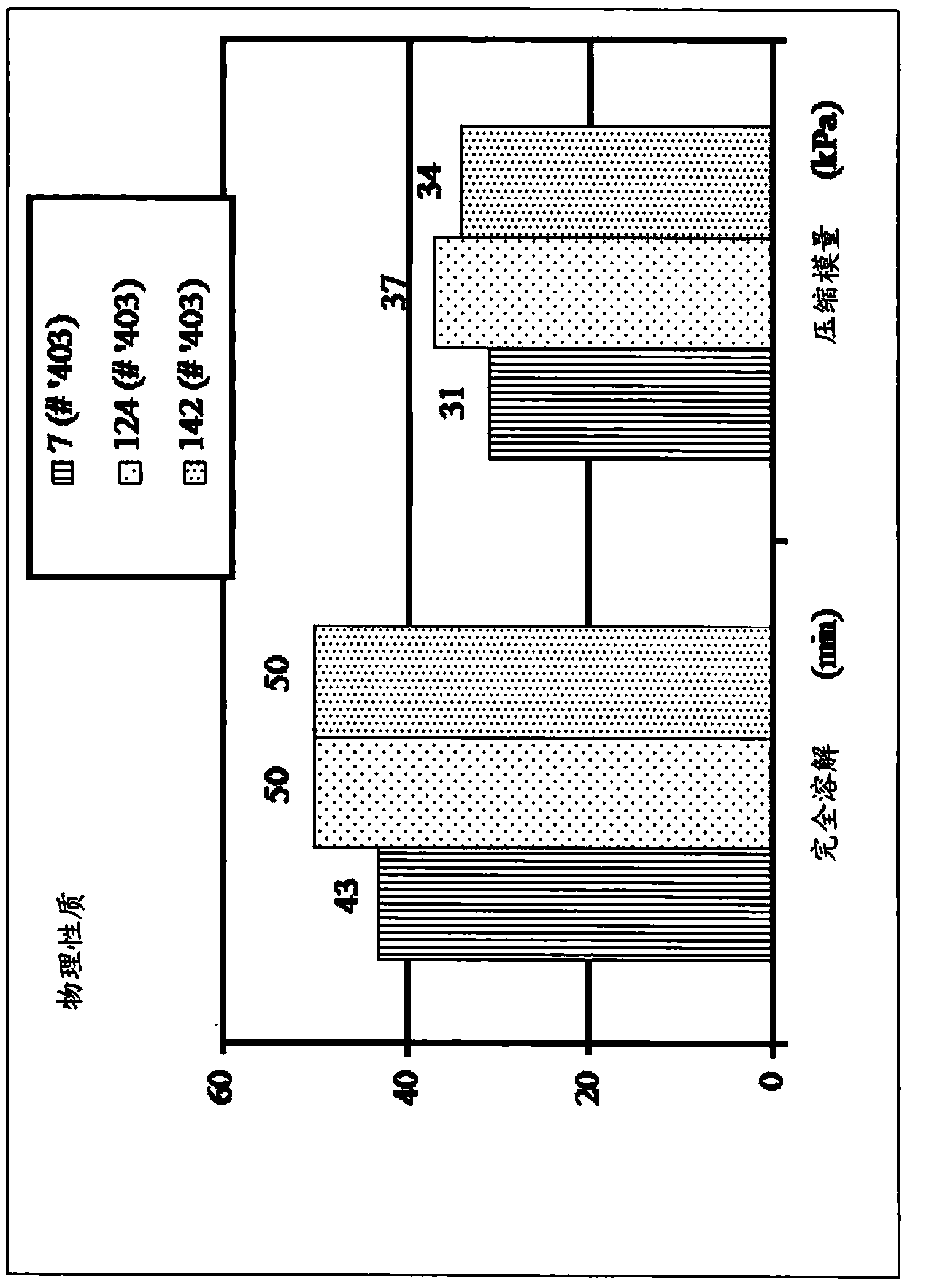
Examples
preparation example Construction
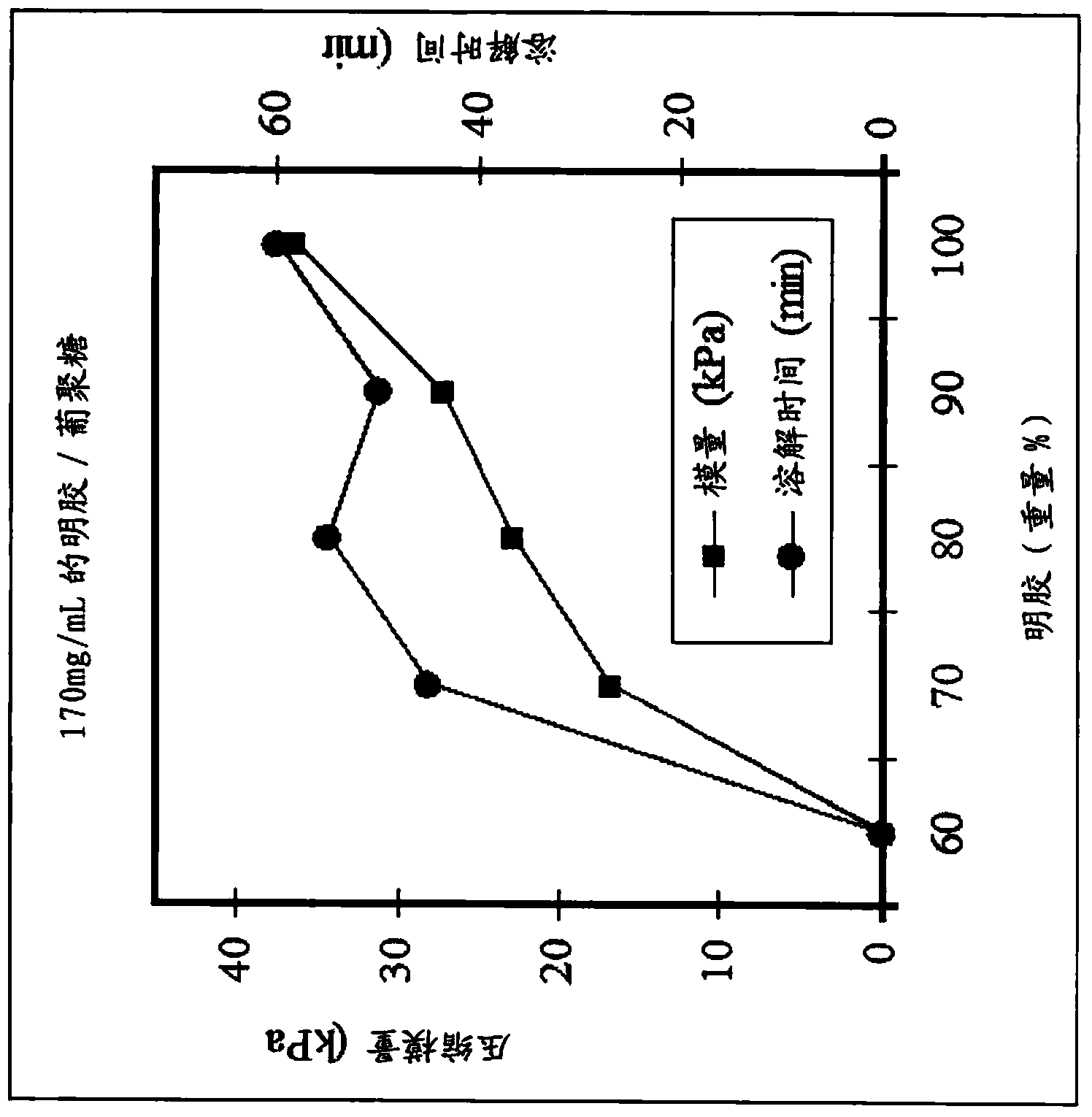
[0051] In preparing the compositions of the present disclosure, the polymer components can be mixed together with sufficient water (or other solvent) to allow the polymers to interact. The polymers can be added to a dry mixing vessel and then hydrated together, or separate solutions of the individual polymers can be prepared and then mixed together. Buffers or titrants may be added to adjust pH and ionic strength. The combination of components can then be mixed under heating (eg, to a temperature greater than the liquid transition temperature discussed herein) to form a homogeneous composition. During processing, specific methods can be used to tailor the composition to further enhance desired properties.
[0052] To facilitate the stability of the resulting gel matrix, it may be desirable to concentrate in situ the fraction of the polymer that contributes to the stability of the final composition. The method may be used in the production of the composition to remove the mos...
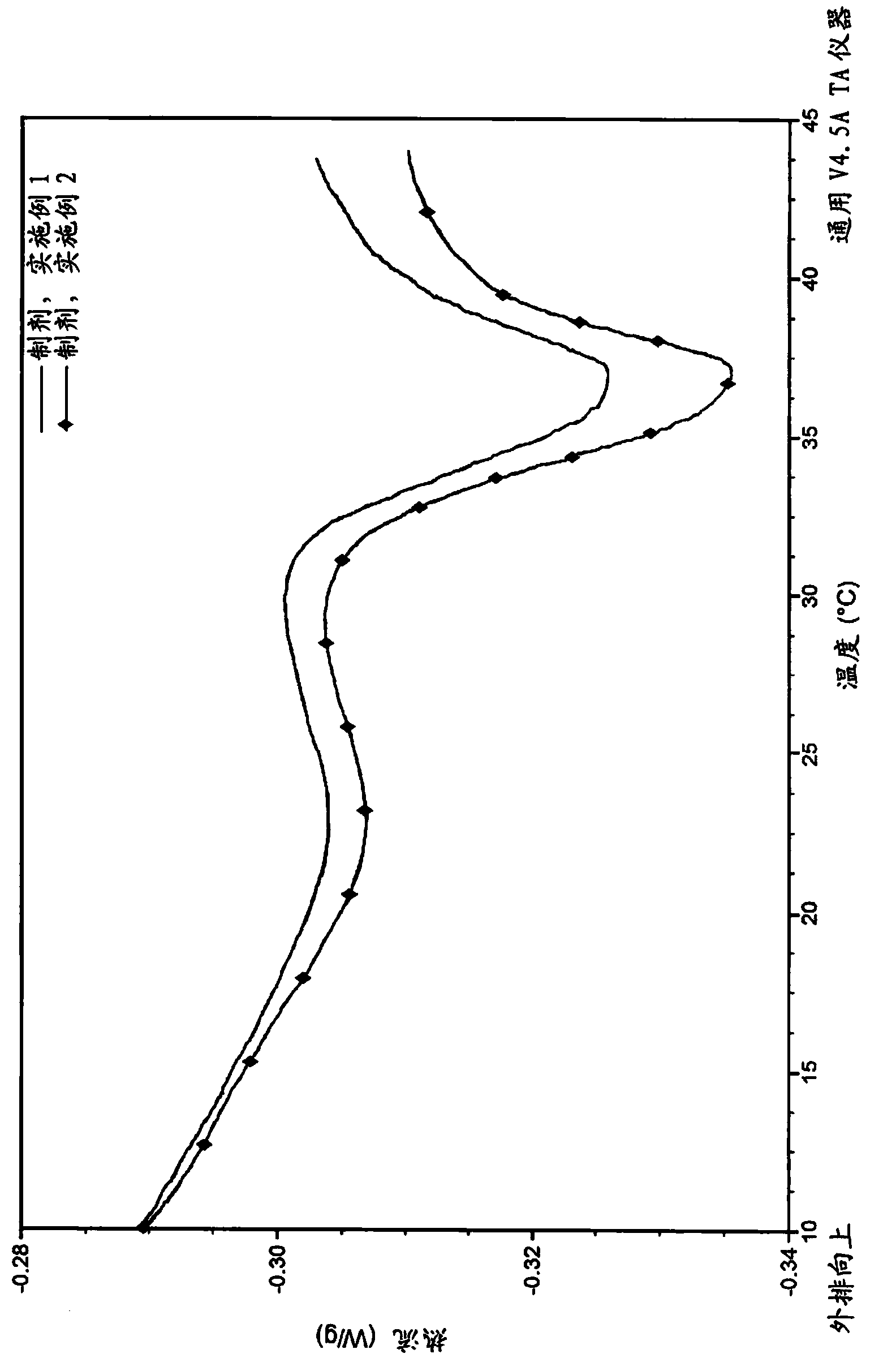
Embodiment 1
[0070] Example 1. Preparation of gelatin / dextran composition
[0071] The composition according to one embodiment of the present disclosure was prepared by sequentially adding the liquid and powder materials (Table 1 ) at elevated temperature at specific time points while mixing to maintain a liquid homogeneous state. The solution is then sterile filtered, aseptically dispensed into vials, sealed, and stored at refrigerated temperature.
[0072] Table 1
[0073]
[0074]
[0075] Glucose and medium 199 were weighed and transferred to a pre-heated water-jacketed (50°C) glass vessel and mixed using a stir bar. After L-cysteine, L-alanyl-L-glutamine, L-glutamic acid, L-lysine, and disodium EDTA were dispensed into the vessel, the mixture was equilibrated with medium 199 to 50°C. After the equilibration time, the dextran powder was added and the components were allowed to mix. Once the dextran is in solution, gelatin is added to the container and allowed to dissolve. O...
Embodiment 2
[0077] Example 2. Preparation of Gelatin / Dextran Compositions with Phosphate Buffer
[0078] Using the process described in Example 1, compositions were prepared by sequentially adding the liquid and powder materials at elevated temperatures while mixing to maintain a liquid homogeneous state. The solution is then sterile filtered and dispensed aseptically. Compositions were prepared by first placing 2,282 ml of phosphate-buffered saline (PBS) in a pre-heated water-jacketed (50° C.) glass vessel and mixing using a stir bar. The mixture was equilibrated with PBS to 50° C. after 375 μl L-cysteine, 6.9 ml L-glutamate, 13.8 ml L-lysine and 24.2 ml disodium EDTA were dispensed into the container. After equilibration time, 137.5 grams of dextran powder was added and the components were mixed. Once the dextran was in solution, 330 grams of gelatin was added to the container and allowed to dissolve. Once the gelatin was in solution, 10% sodium hydroxide was added to adjust the pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com