Induced malignant stem cells
A stem cell and malignant technology, applied in the field of induced malignant stem cells that proliferate, can solve the problems of difficult to maintain abnormality, chromosomal abnormalities, impossible target/compound screening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
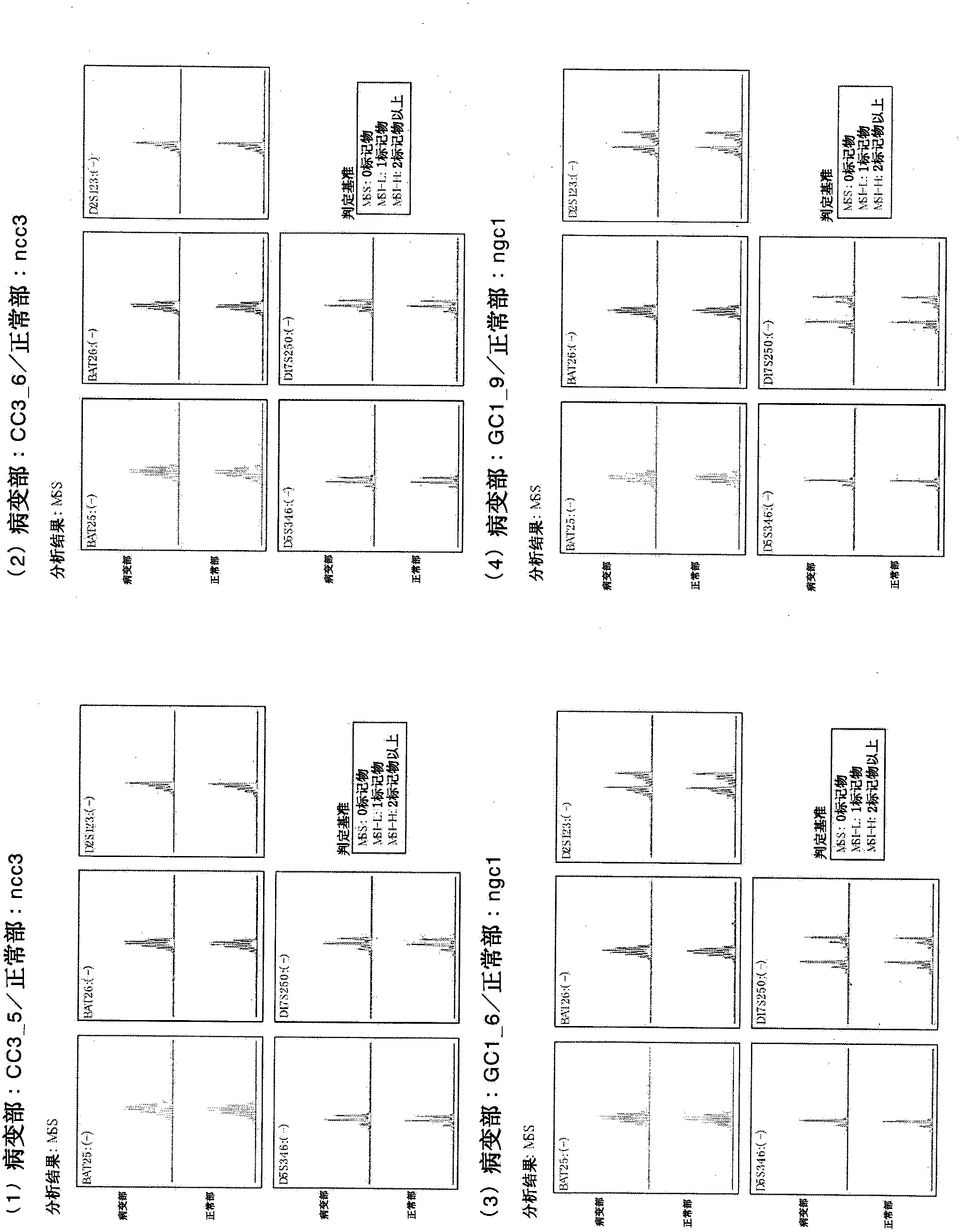
[0307] Example 1: Preparation and induction of malignancy from cells (GC2) derived from cancer tissues of gastric cancer patients stem cell
[0308] From the donor No. 1 human gastric cancer patient (medical information: gastric cancer, female, 67 years old, Japan) stored in a preservation solution (Hank's solution with kanamycin and amphotericin) refrigerated and transported for several hours Human, blood type O, no chemotherapy, no radiotherapy, no immunosuppressive therapy, no history of smoking, no history of drinking, no drug poisoning, no medical treatment, HIV negative, HCV negative, HBV negative, syphilis negative ) Of fresh cancer tissue isolated cells (GC2). Likewise, cells were isolated from fresh non-cancerous tissue (NGC2). To the obtained cells derived from gastric cancer tissue as solid cancer, 4 genes (POU5F1, KLF4, SOX2, c-Myc) Sendai virus vector solution of CytoTune iPS (DV-0301-1) manufactured by DNAVEC Corporation were added to perform gene Introduced, th...
Embodiment 2
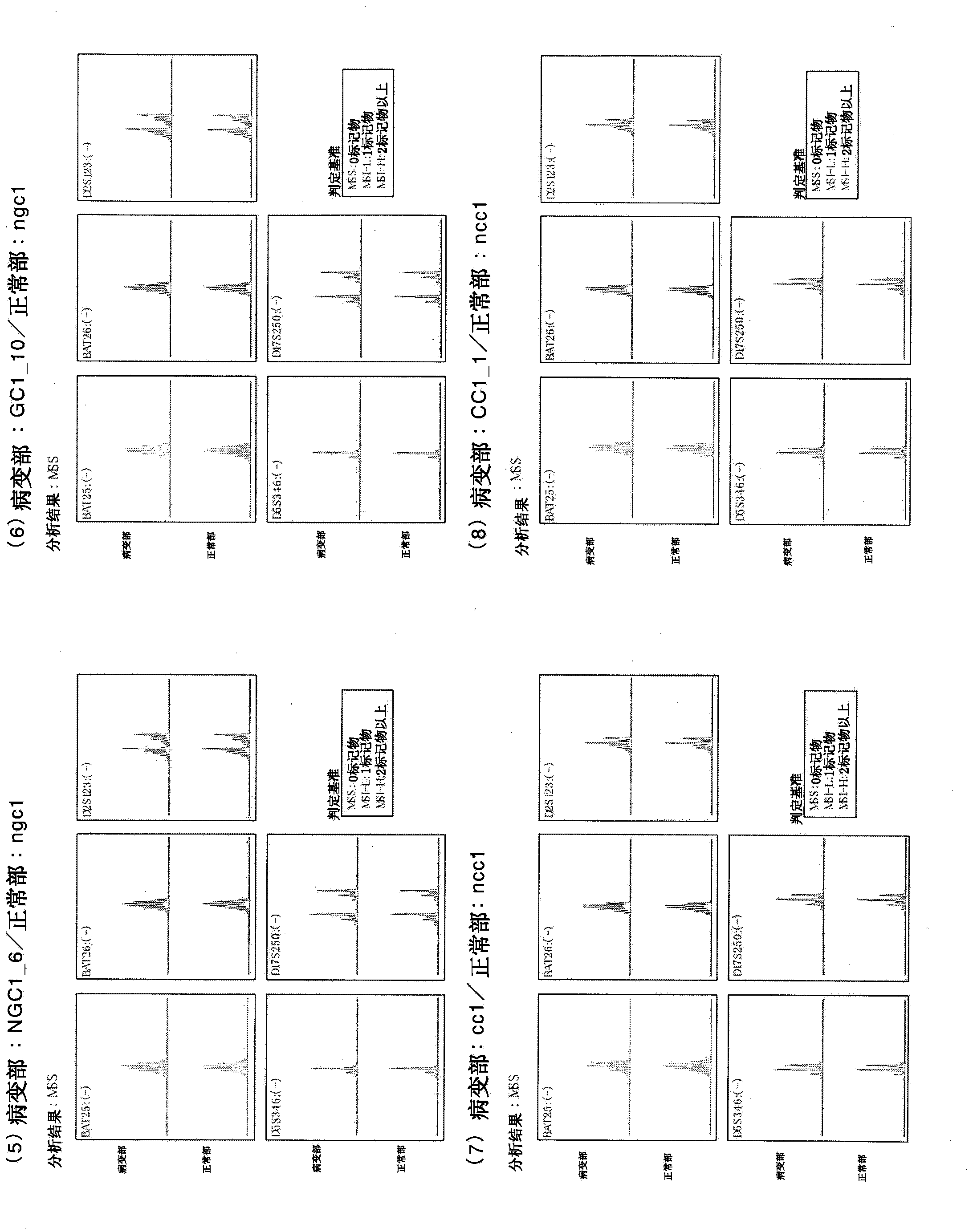
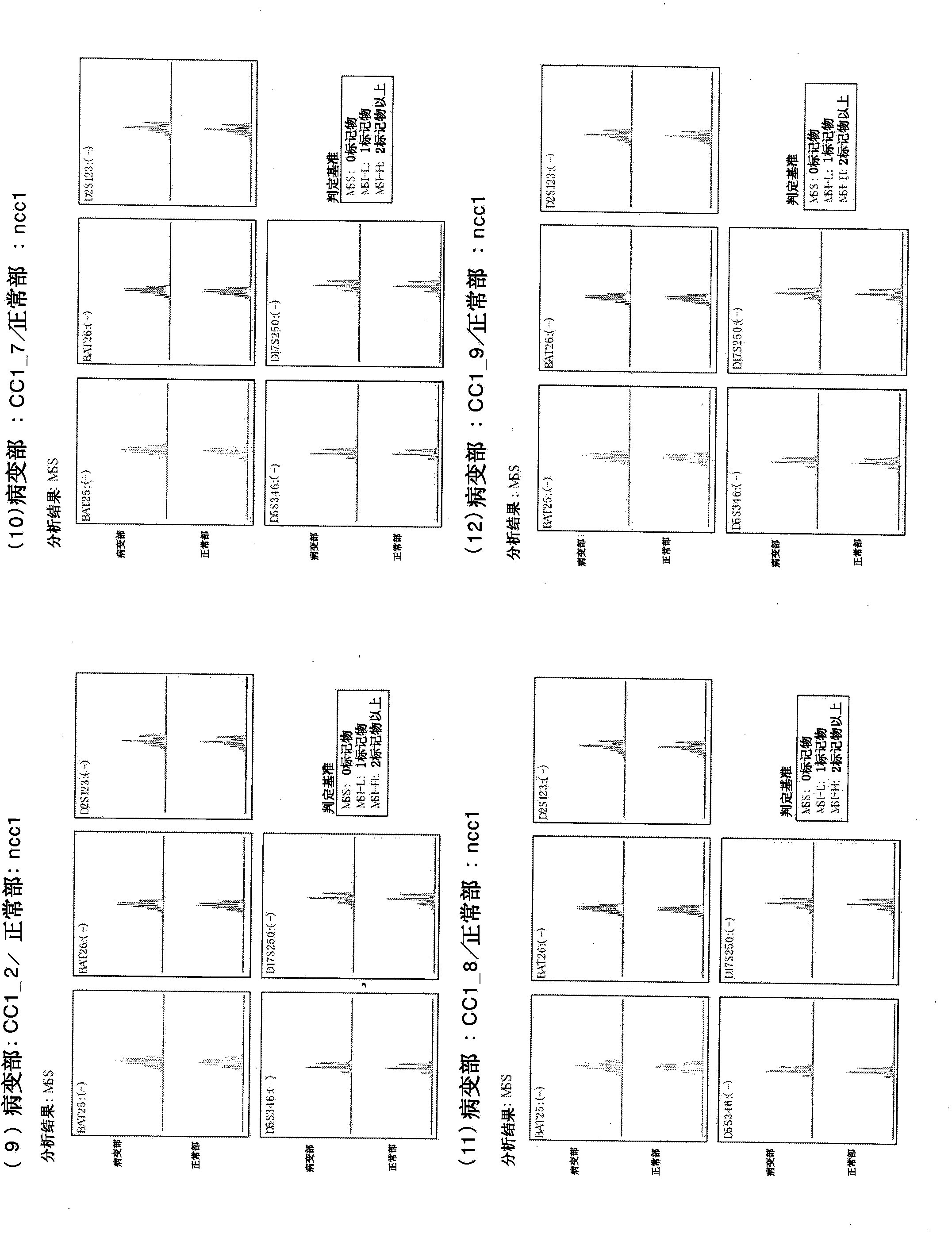
[0322] Example 2: Producer induction from cells (CC3) derived from cancer tissues of patients with colorectal cancer Malignant stem cells
[0323] From donor No. 2 human colorectal cancer patients (medical information: sigmoid colon cancer, male, 77) who were refrigerated and transported for several hours in the preservation solution (Hank's solution with kanamycin and amphotericin) Years old, Japanese, blood type A, no chemotherapy, no radiotherapy, no immunosuppressive therapy, no history of smoking, history of drinking beer 1 bottle / day, no drug poisoning, no drug treatment, HIV negative, HCV Negative, HBV-negative, syphilis-negative) fresh cancer tissue isolated cells (CC3). Similarly, cells were isolated from fresh non-cancerous tissue from the same donor (NCC3). To the obtained cells derived from cancer tissues of human colorectal cancer patients, 4 genes (POU5F1, KLF4, SOX2, c-Myc) Sendai virus vector solution of CytoTune iPS (DV-0301-1) manufactured by DNAVEC Co., Ltd....
Embodiment 3
[0333] Example 3: Preparation of retroviral vector
[0334] Using Fugene HD (manufactured by Roche; Cat no.4709691), a retroviral vector plasmid containing the 3 genes of POU5F1-pMXs, KLF4-pMXs, and SOX2-pMXs was introduced into Plat as a packaging cell for the preparation of pan-tropic retroviral vectors -In GP cells, prepare retroviral vector solution. The details are as follows.
[0335]
[0336] POU5F1-pMXs, KLF4-pMXs, SOX2-pMXs were constructed vectors (Table 9).
[0337] The amount of each vector is POU5F1-pMXs 5μg, KLF4-pMXs 2.5μg, SOX2-pMXs 1.25μg, Venus-pCS2 1.25μg, VSV-G-pCMV 5μg, GFP-pMXs1.25μg (manufactured by Cell Biolab), FuGENE HD 45μl .
[0338]
[0339] The genes POU5F1-pMXs, KLF4-pMXs and SOX2-pMXs were constructed vectors (Table 9).
[0340] The amount of each vector was POU5F1-pMXs 5 μg, KLF4-pMXs 2.5 μg, SOX2-pMXs 1.25 μg, Venus-pCS2 1.25 μg, VSV-G-pCMV 5 μg, GFP-pMXs 1.25 μg, and FuGENE HD 45 μg.
[0341]
[0342] The genes POU5F1-pMXs, KLF4-pMXs, SOX2-pMXs were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com