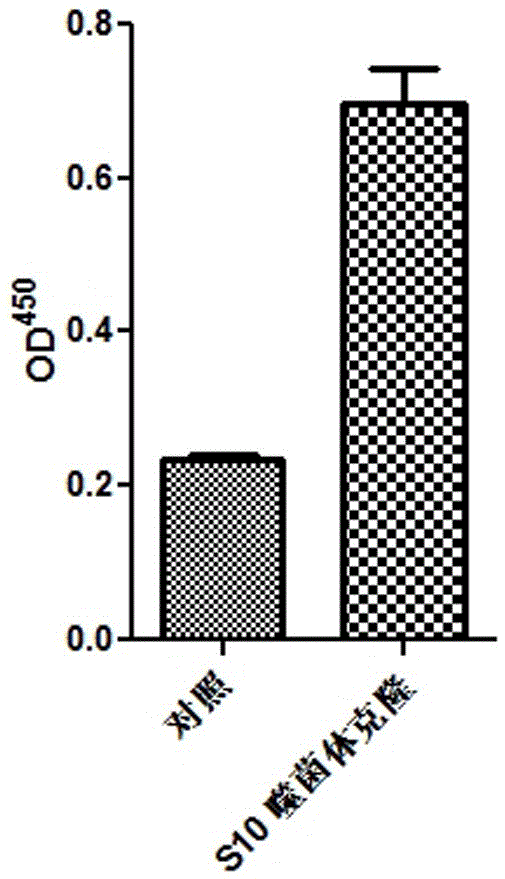
PD-l1 IgV affinity peptide S10 with antitumor activity
An anti-tumor activity and affinity technology, used in anti-tumor drugs, peptides, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The anti-tumor activity targeting PD-L1IgV affinity peptide S10 provided by the present invention specifically binds to the PD-L1IgV region, and is screened by phage display peptide library technology, and its amino acid sequence is:
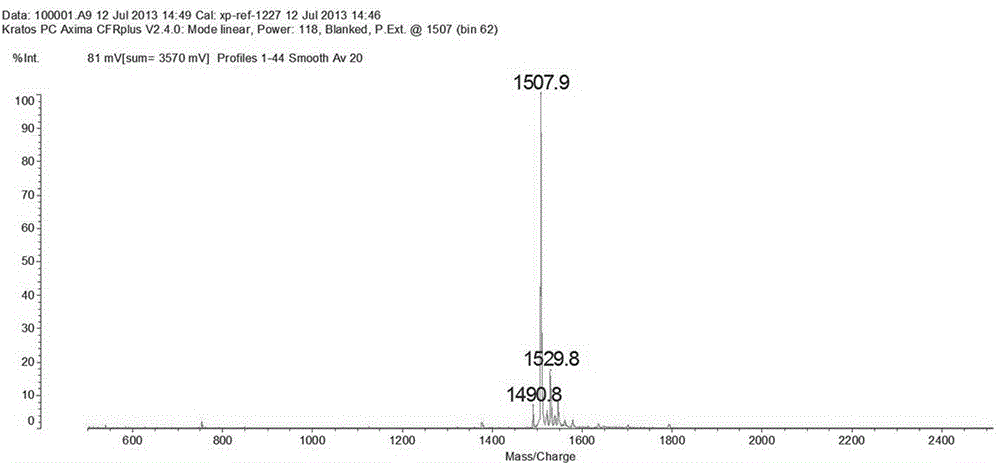
[0021] Trp-Ser-His-Gly-Gly-His-Gln-His-Phe-Ile-Arg-Phe, namely W-S-H-G-G-H-Q-H-FI-R-F, has a molecular weight of 1507.7.
[0022] Due to the high cost of monoclonal antibodies and the inability of large-scale production, we selected the PD-L1IgV region protein purified by prokaryotic expression as the target, and screened the peptides that can specifically bind to PD-L1IgV by phage display technology. To block PD-1 / PD-L1 signaling pathway. For the convenience of those skilled in the art to implement the present invention, a brief description of its screening process is as follows:
[0023] The preparation instructions for the media and main solutions used in the screening process are as follows:
[0024] LB liquid medium: Weigh 10 g of ...
Embodiment 2
[0076] The affinity peptide S10 of PD-L1IgV with anti-tumor activity is synthesized by Fomc solid-phase peptide synthesis method, and the synthesis steps are briefly described as follows:
[0077] The main reagents used in the synthesis process are:
[0078] Heading liquid: acetic anhydride / pyridine solution (1:1, v / v);
[0079] Indene detection reagent: A. Ninhydrin / ethanol solution (5%, w / v)
[0080] B. Phenol / Ethanol (4:1, w / v)
[0081] C. Potassium cyanide / pyridine (2%, v / v)
[0082] Deprotection solution: piperidine / DMF solution (20%, v / v);
[0083] Cleavage reagent: by volume, TFA (82.5%), H 2 O (5%), phenol (5%), thioanisole (5%), ethanedithiol (2.5%).
[0084] The synthetic steps are briefly described as follows:
[0085] (1) Swell the resin, add the first amino acid
[0086] A. Swelling resin: Take 0.3~0.5g of Rink resin (the C-terminal amino acid of the peptide connected to the resin is an amide) and place it in a cleaned and dried peptide synthesizer, add an...
Embodiment 3
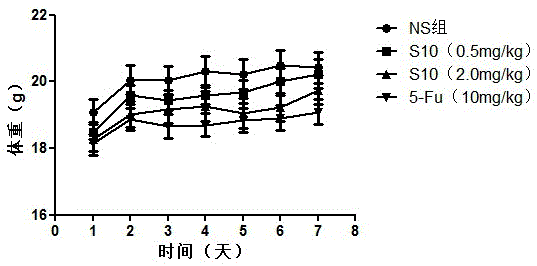
[0128] Taking the PD-L1IgV affinity peptide S10 with anti-tumor activity prepared in Example 2 as an example, the inventors conducted further in vivo experiments on tumor-bearing mice, and the specific experimental procedures were as follows:
[0129] (1) Affinity peptide S10 inhibits the growth of transplanted tumors in mice bearing CT26 colon cancer
[0130] Select 20 experimental Balb / c mice, adjust the cell concentration to 5×10 mouse colon cancer (CT26) cells with normal saline (NS) 6 cells / mL, 0.1mL cell suspension (containing 5×10 5 cells) were inoculated subcutaneously in the armpit of the right forelimb of each Balb / c mouse, and the growth of the subcutaneous tumor was continuously observed.
[0131] The affinity peptide S10 prepared in Example 2 was dissolved in physiological saline to prepare a polypeptide drug, which was subpackaged and stored at -20°C for future use.
[0132] Nine days after being inoculated with mouse-derived colon cancer (CT26) cells, the mice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com