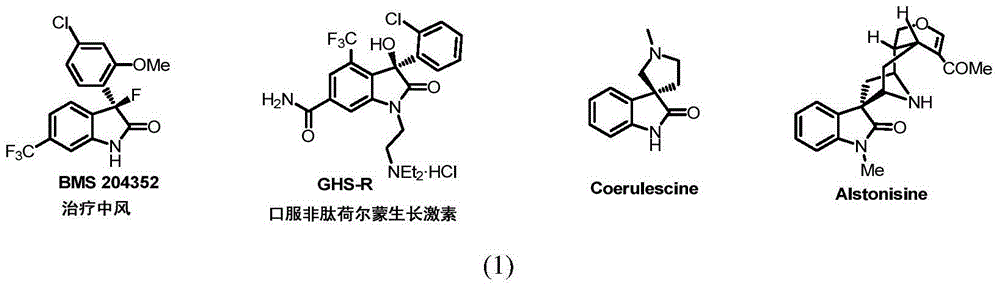
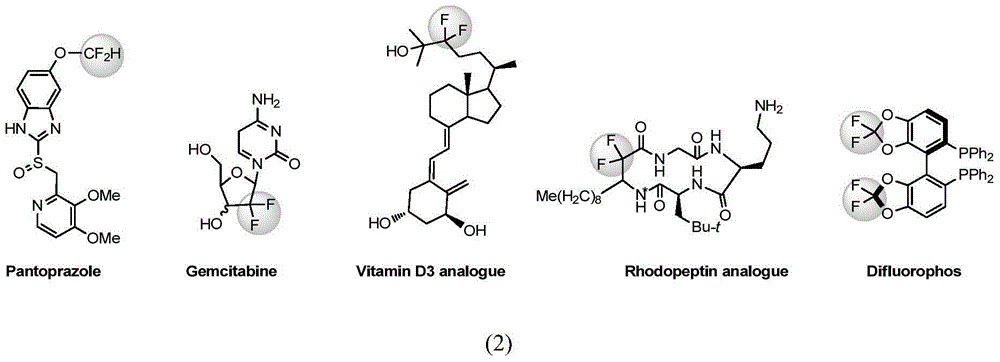
3-difluoro alkyl substituted all-carbon quaternary carbon oxoindole derivative and synthetic method thereof
A technology of carboxy indole derivatives and difluoroalkyl, which is applied in the field of all-carbon quaternary carboxy indole derivatives and their synthesis, and achieves the effects of high yield and enantioselectivity, wide application range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of 3-difluoroalkyl-substituted oxindole III-1:
[0041]
[0042] In a 5.0mL reaction flask, add dinitrile-substituted alkenyl oxide indole I-1 (48.8mg, 0.25mmol), phenyl-substituted difluoroenolsilyl ether II-1 (570mg, 2.5mmol), distilled water (5.0 mL), the reaction solution was stirred at 50° C. for 15 h. TLC detects that the raw materials have basically reacted, and the reaction is stopped. The reaction solution was extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, transferred to a 50mL round-bottomed flask, added about 1.0g of crude silica gel, spin-dried and then column chromatographed, eluent (ethyl acetate / petroleum ether=1 / 3), the product III-1 was obtained as light yellow solid 80.1mg, and the yield was 90%. 1 H NMR (400MHz, CDCl 3 ):δ8.49(s,1H),8.02-8.00(m,2H),7.68-7.64(m,2H),7.50-7.42(m,3H),7.18-7.14(m,1H),7.06-7.04 (m,1H),4.92(s,1H); 13 C NMR (100MHz, CDCl 3 ): δ185.3(t, J=30Hz, 1C), 170.5(d, J=8Hz, 1C), 141.7, 13...
Embodiment 2
[0044] Synthesis of 3-difluoroalkyl-substituted oxindole III-2:
[0045]
[0046] Add benzoic acid (3.1mg, 0.025mmol), dinitrile-substituted alkenyl oxide indole I-2 (53.3mg, 0.25mmol) and phenyl-substituted difluoroenolsilyl ether II to a 5.0mL reaction flask in sequence -1 (85.5mg, 0.375mmol), tetrahydrofuran (3.0mL); the reaction solution was stirred at room temperature for 5h. TLC detects that the raw material has basically reacted completely, and the reaction is stopped. Transfer the reaction solution to a 50mL round-bottomed flask, drain the solvent under the oil pump, perform direct column chromatography, eluent (ethyl acetate / petroleum ether=1 / 3), and obtain product III-2 as a light yellow solid 85.8 mg, yield 93%. 1 H NMR (400MHz, CDCl 3 ):δ8.34(s,1H),8.03-8.01(m,2H),7.71-7.67(m,1H),7.52-7.49(m,2H),7.43-7.42(m,1H),7.20-7.15 (m,1H),7.04-7.01(m,1H),4.90(s,1H); 13 C NMR (100MHz, CDCl 3 ): δ185.2(t, J=31Hz, 1C), 170.5(d, J=8Hz, 1C), 159.2(d, J=243Hz, 1C), 137.8(d...
Embodiment 3
[0048] Synthesis of 3-difluoroalkyl-substituted oxindole III-3:
[0049]
[0050] Add Cu(OTf) sequentially to the 5.0mL reaction flask 2 (4.5mg, 0.0125mmol), dinitrile substituted alkenyl oxide indole I-3 (52.3mg, 0.25mmol), phenyl substituted difluoroenol silyl ether II-1 (62.7mg, 0.275mmol), Anhydrous ether (2.0 mL); the reaction solution was stirred at room temperature for 3 h. TLC detects that the raw material has basically reacted completely, and the reaction is stopped. Transfer the reaction solution to a 50mL round-bottomed flask, drain the solvent under the oil pump, perform direct column chromatography, eluent (ethyl acetate / petroleum ether=1 / 3), and obtain product III-3 as a light yellow solid 77.6 mg, yield 85%. 1 H NMR (400MHz, CDCl 3 ):δ8.14(s,br,1H),8.03-8.01(m,2H),7.68-7.65(m,1H),7.51-7.47(m,3H),7.25-7.24(m,1H),6.95 -6.93(m,1H),4.89(s,1H),2.35(s,3H); 13 C NMR (100MHz, CDCl 3 ): δ185.4(t, J=31Hz, 1C), 170.4, 139.2, 135.4, 133.8, 132.4, 130.4(t, J=3Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com