Method for preparing chirality polyaniline under cyclodextrin induction and biocatalysis in DBSA (dodecylbenzene sulfonic acid) micellar system
A technology of chiral polyaniline and biocatalysis, which is applied in the field of chemistry and biochemistry, can solve the problems of high cost, limited application, easy inactivation, and high price of natural peroxidase, and achieves good chiral effect, low price, good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
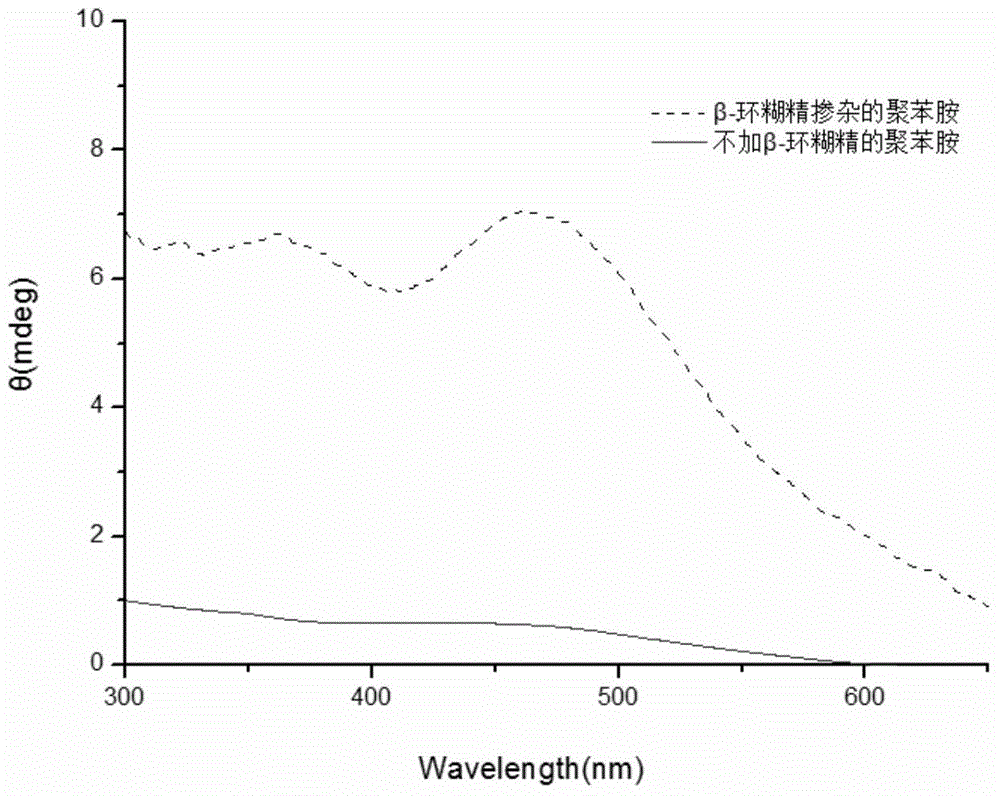
[0029] Weigh 0.05mmol of β-cyclodextrin and dissolve it in 9.7mL of citric acid-disodium hydrogen phosphate buffer solution with pH 2.0; after stirring evenly, add 0.125mmol of aniline monomer and 0.125mmol of DBSA respectively; weigh 2mg of hemoglobin with 239μl Distilled water was dissolved and added to the system; then 61 μL of hydrogen peroxide solution with a concentration of 9.823 mol / L was added to the reaction system. After stirring for 24 hours, an equal volume of methanol was added to break the emulsification and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained. Characterized by circular dichroism chromatography, a strong circular dichroism peak was generated at 475 nm, which proved that it had the characteristics of chiral polyaniline. as follows figure 1 shown.
Embodiment 2
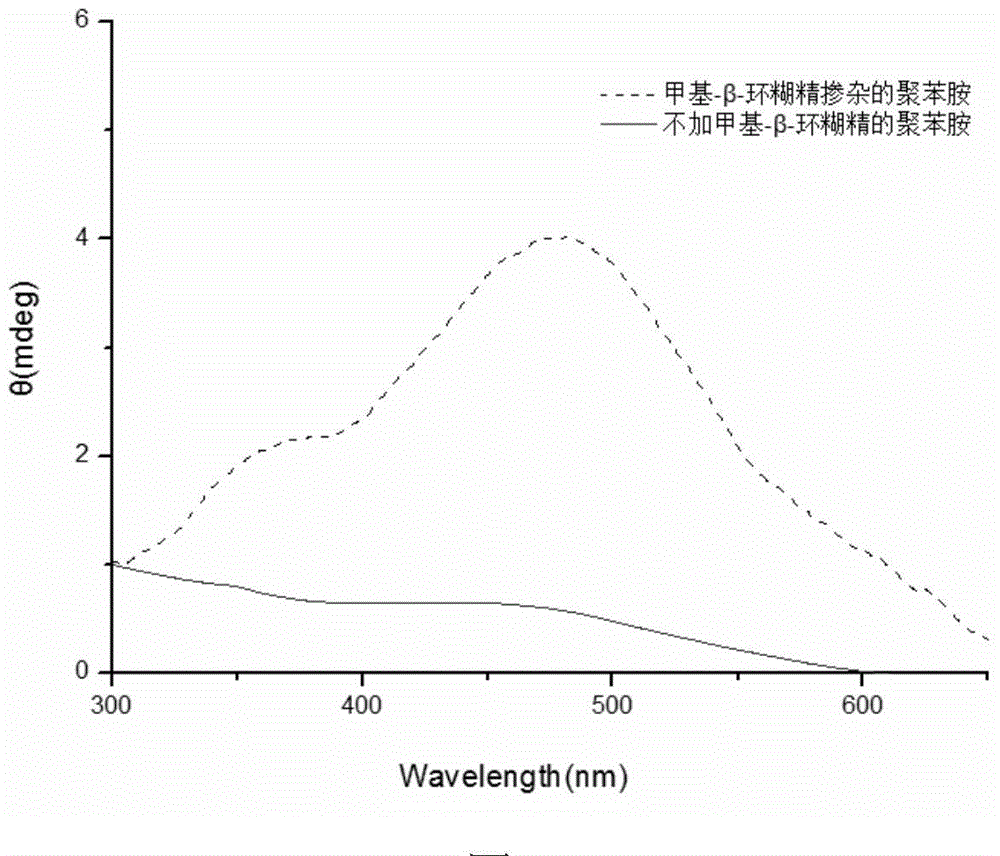
[0031] Weigh 0.1mmol of methyl-β-cyclodextrin and dissolve it in 9.7mL of citric acid-disodium hydrogen phosphate buffer solution with pH 2.0; stir well and add 0.125mmol of aniline monomer and 0.17mmol of DBSA respectively; weigh 3mg The hemoglobin was dissolved in 219 μl of distilled water and added to the system; then 81 μL of hydrogen peroxide solution with a concentration of 9.823 mmol / L was added to the reaction system. After stirring for 24 hours, an equal volume of methanol was added to break the emulsification and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained. Characterized by circular dichroism, a strong circular dichroism peak was generated at 475 nm, which proved that it had the characteristics of chiral polyaniline. as follows figure 2 shown.
Embodiment 3
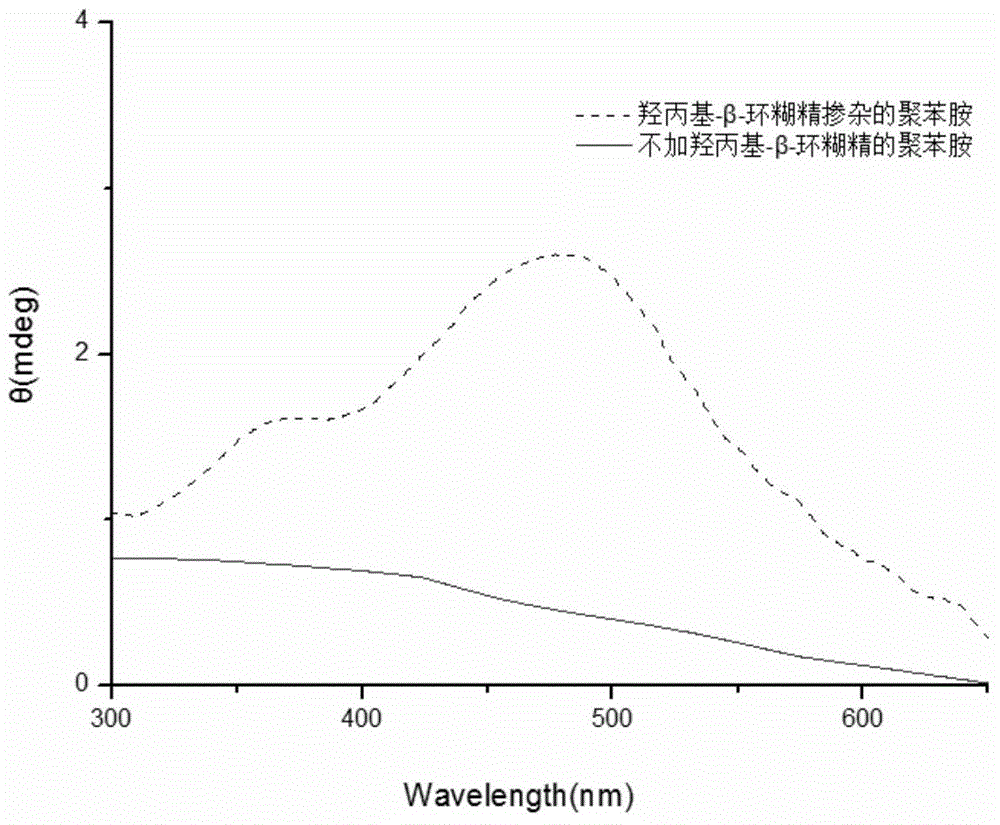
[0033] Weigh 0.2mmol of hydroxypropyl-β-cyclodextrin and dissolve it in 9.7mL of citric acid-disodium hydrogen phosphate buffer solution with pH 2.0; after stirring evenly, add 0.125mmol of aniline monomer and 0.25mmol of DBSA respectively; weigh 6 mg of hemoglobin was dissolved in 204 μl of distilled water and added to the system; then 96 μL of hydrogen peroxide solution with a concentration of 9.823 mol / L was added to the reaction system. After stirring for 24 hours, an equal volume of methanol was added to break the emulsification and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained. Characterized by circular dichroism, a strong circular dichroism peak was generated at 475 nm, which proved that it had the characteristics of chiral polyaniline. as follows image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com