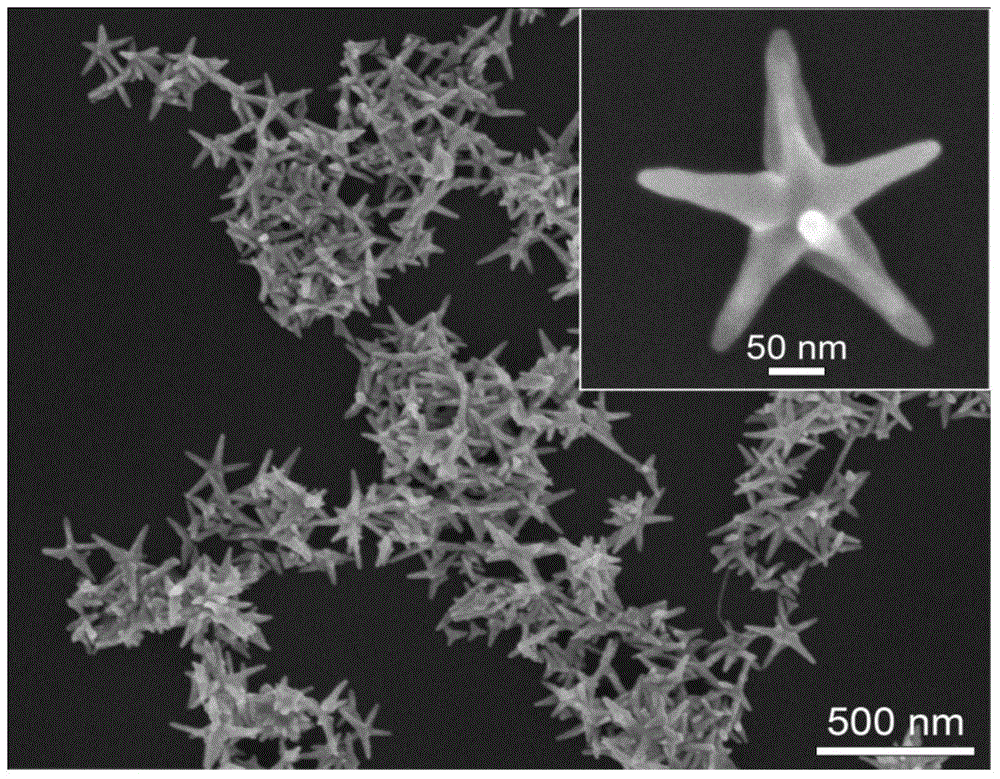
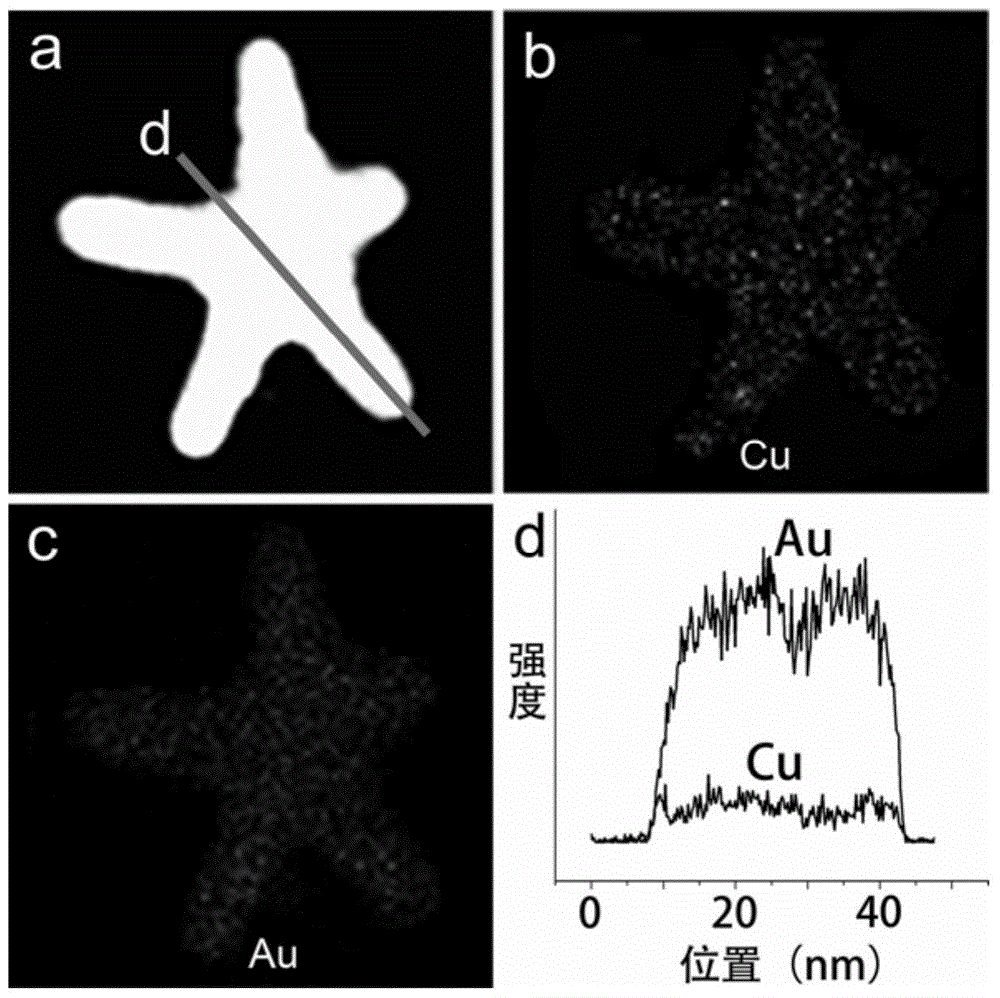
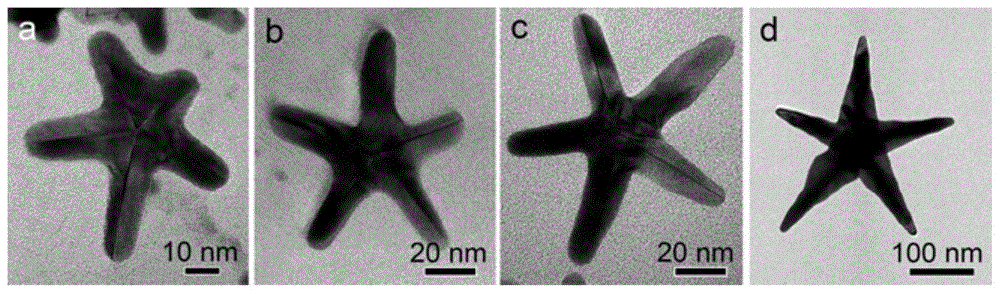
Preparation method of pentagram-shaped au-cu alloy nanocrystals and products prepared therefrom
A pentagram-shaped, alloy nanotechnology, applied in crystal growth, nanotechnology, chemical instruments and methods, etc., can solve the problems of large-scale preparation problems, high costs, and high experimental costs, and achieve large-scale preparation and broad application prospects. , the effect of short operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of pentagram-shaped Au-Cu alloy nanocrystals with an average size of 200 nm:
[0023] At room temperature, add CuCl with a concentration of 100mM to a 20ml glass reaction flask in sequence 2 ·2H 2 O aqueous solution 0.3mL, the concentration is 100mM HAuCl 4 ·3H 2 0.3 mL of O aqueous solution, 45 mg of HDA, 0.28 mL of 1 M aqueous glucose solution, 4 mL of deionized water, and one magneton. After capping the bottle tightly, stir on a magnetic stirrer at room temperature overnight. After that, it was transferred to an oil bath at 100° C., heated and reacted with magnetic stirring for 30 min. During the reaction, the color of the solution in the bottle changed from yellow-green to dark brown to obtain a crude product. Take out the reaction bottle, and after it cools to room temperature, transfer the reaction solution in the bottle to a centrifuge tube and centrifuge at 10,000 rpm for 8 min; under the same conditions, wash with deionized water and ethanol for...
Embodiment 2
[0025] Preparation of pentagram-shaped Au-Cu alloy nanocrystals with an average size of 100 nm:
[0026] At room temperature, add CuCl with a concentration of 100mM to a 20ml glass reaction flask in sequence 2 ·2H 2 O aqueous solution 0.3mL, the concentration is 100mM HAuCl 4 ·3H 2 0.3 mL of O aqueous solution, 45 mg of HDA, 0.28 mL of 1 M aqueous glucose solution, 4 mL of deionized water, and one magneton. After capping the bottle tightly, stir on a magnetic stirrer at room temperature overnight. After that, it was transferred to an oil bath at 100 °C, heated and reacted with magnetic stirring for 5 min, and then 30 mg of HDA was added. The bottle was cooled with ice water. After the temperature dropped to room temperature, the reaction solution in the bottle was transferred to a centrifuge tube and centrifuged at 10,000 rpm for 8 min; to wash off residual reactants, the encapsulating agent HDA and the reducing agent glucose. Finally, clean pentagram-shaped Au-Cu alloy ...
Embodiment 3
[0028] Preparation of pentagram-shaped Au-Cu alloy nanocrystals with an average size of 70 nm:
[0029] At room temperature, add CuCl with a concentration of 100mM to a 20ml glass reaction flask in sequence 2 ·2H 2 O aqueous solution 0.3mL, the concentration is 100mM HAuCl 4 ·3H 2 0.3 mL of O aqueous solution, 45 mg of HDA, 0.28 mL of 1 M aqueous glucose solution, 4 mL of deionized water, and one magneton. After capping the bottle tightly, stir on a magnetic stirrer at room temperature overnight. After that, it was transferred to an oil bath at 100 °C, heated and reacted with magnetic stirring for 4 min, then 30 mg of HDA was added, and the reaction flask was still heated in the oil bath for reaction. The bottle was cooled with ice water. After the temperature dropped to room temperature, the reaction solution in the bottle was transferred to a centrifuge tube and centrifuged at 10,000 rpm for 8 min; to wash off residual reactants, the encapsulating agent HDA and the redu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com