Method for slow release of hydrogen by use of solid sodium borohydride and crystalline hydrate
A technology of crystalline hydrate and sodium borohydride, which is applied in the field of hydrogen storage and hydrogen production, can solve the problems of poor durability of catalysts, potential safety hazards of hydrogen accumulation, and attenuation of catalyst activity, and meet the requirements of non-polluting products, easy availability of raw materials, and safety requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Comparison of hydrogen production performance of two kinds of hydrogen production systems in embodiment 1
[0032] Construction of hydrogen production system:
[0033] (1) Solid fuel: 1g NaBH 4 Powder + 0.005g NaOH powder, mix evenly;
[0034] (2) Water source
[0035] (a) 2 g of water;
[0036] (b) 3.36g Na 2 CO 3 10H 2 O powder.
[0037] Hydrogen production method:
[0038] The solid fuel is placed in a closed reaction chamber, and then water or crystal hydrate is added to the reaction chamber to fully contact the reactants.
[0039] The performance of the hydrogen production system was tested by the drainage method, and the hydrogen production performance test results:
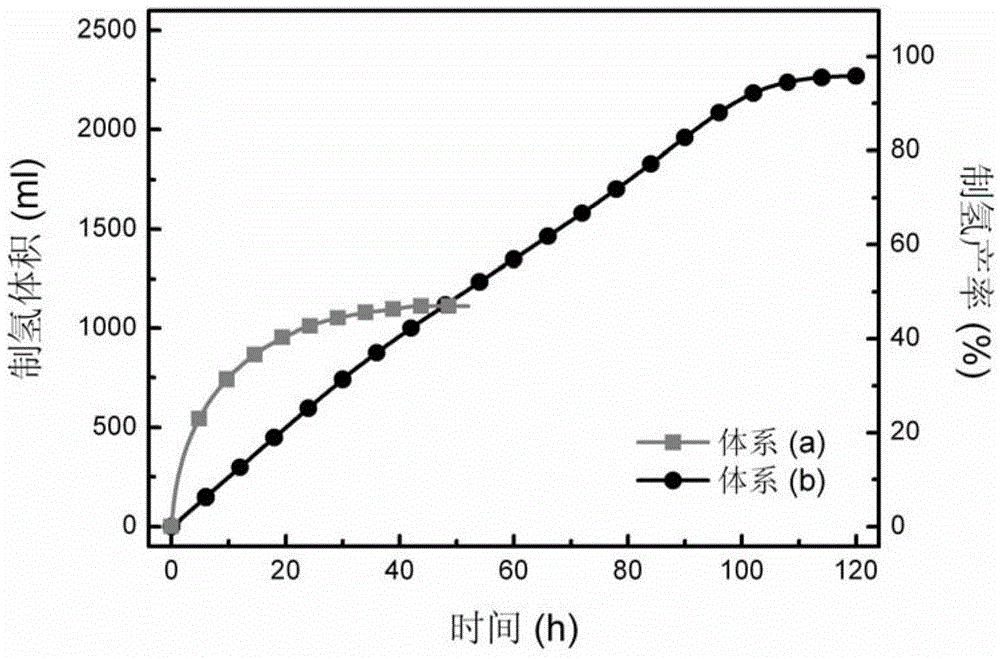
[0040] figure 1 gives water and crystalline hydrates (Na 2 CO 3 10H 2 O) is the comparison of the influence of water source on the performance of the hydrogen production system. It can be seen from the figure that water or Na 2 CO 3 10H 2 The reaction started immediately after O was ad...
Embodiment 2
[0042] Embodiment 2 crystal hydrate species to solid NaBH 4 Effect of hydrolysis hydrogen production system on performance
[0043] Construction of hydrogen production system:
[0044] (1) Solid fuel: 1g NaBH 4 powder + 0.005g NaOH powder;
[0045] (2) Crystalline hydrate:
[0046] (a) 3.78g Na 2 SO 4 10H 2 O powder;
[0047] (b) 3.36g Na 2 CO 3 10H 2 O powder;
[0048] (c) 7.28g NaBO 2 4H 2 O powder.
[0049] The hydrogen production method and the hydrogen production performance test method are the same as in Example 1.
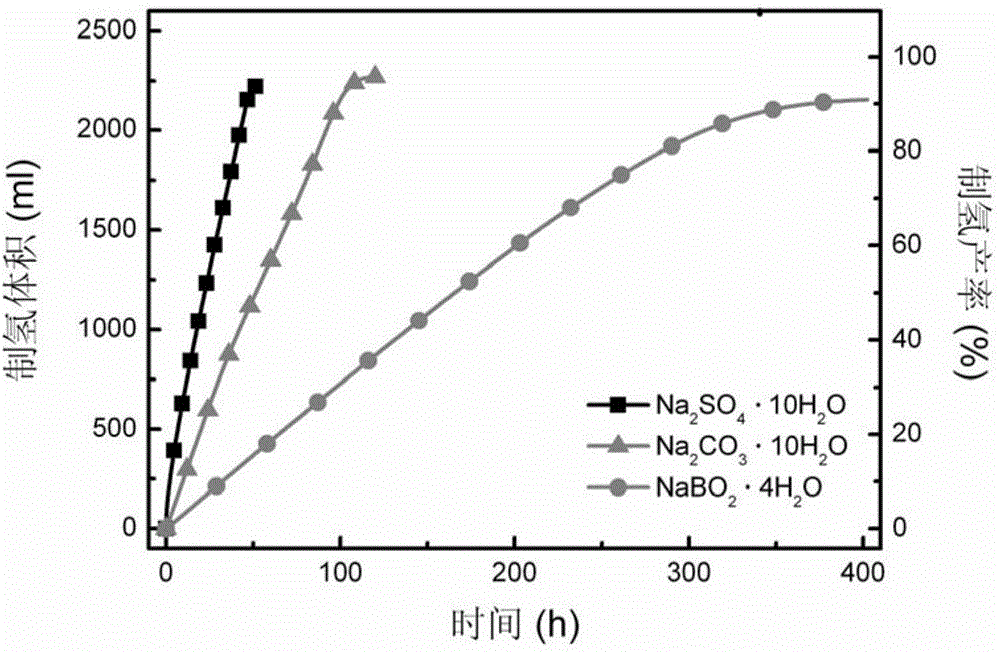
[0050] figure 2 gives different crystalline hydrates versus solid NaBH 4 Performance impact of hydrolysis hydrogen production system. It can be seen from the figure that at room temperature, using Na 2 SO 4 10H 2 O, Na 2 CO 3 10H 2 O and NaBO 2 4H 2 The hydrogen production rate of the O system is stable, the hydrogen production yield is higher than 90%, and the corresponding hydrogen storage densities are 4.4wt%, 4.8wt% and 2.5wt%. ...
Embodiment 3
[0051] The content of embodiment 3NaOH is to solid NaBH 4 Effect of hydrolysis hydrogen production system on performance
[0052] The construction of the hydrogen production system, the hydrogen production method and the hydrogen production performance test method are the same as in Example 1.
[0053] Construction of hydrogen production system:
[0054] (1) Solid fuel: 1g NaBH 4 Powder+x g NaOH powder (x=0, 0.005g, 0.01g, 0.02g);
[0055] (2) Crystal hydrate: 3.78g Na 2 SO 4 10H 2 O powder.
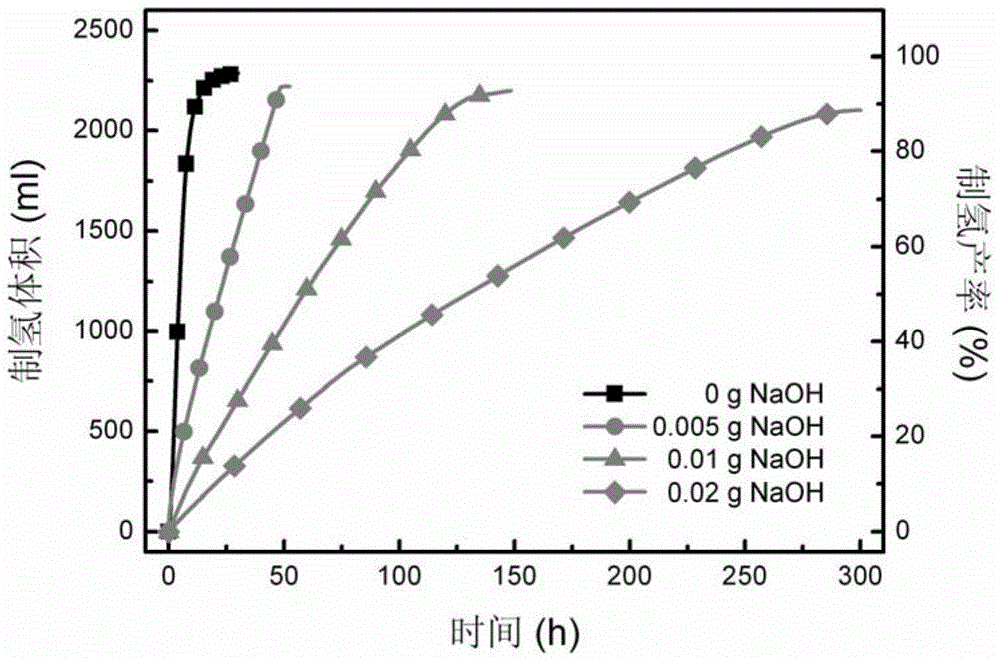
[0056] image 3 gives the NaOH content versus solid NaBH 4 Performance impact of hydrolysis hydrogen production system. It can be seen from the figure that the rate of hydrogen production increases with NaBH 4 Decrease with the increase of NaOH content in the powder. The test results show that the solid NaBH can be regulated by changing the content of NaOH powder 4 The hydrogen production rate of the hydrolysis hydrogen production system.
[0057] The result of embodiment sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com