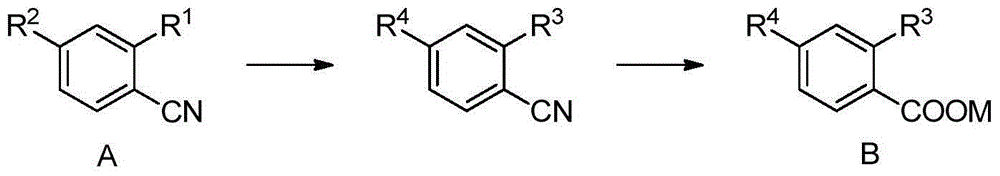
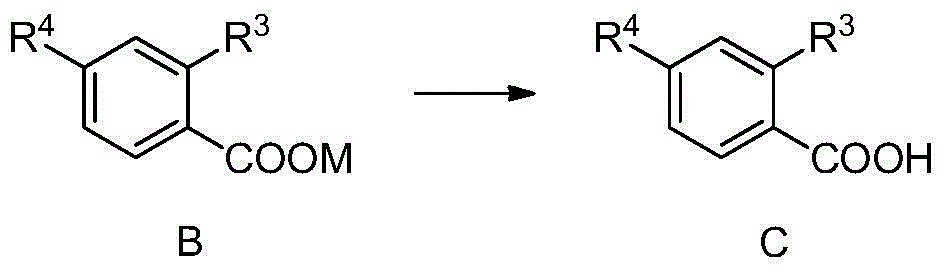
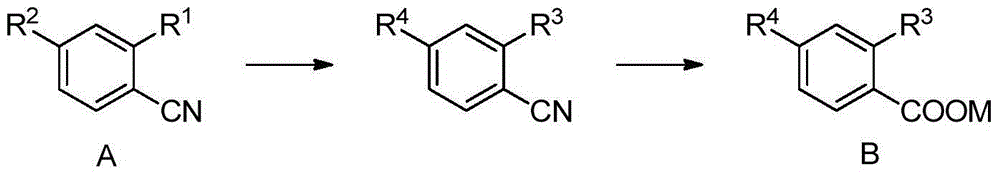
Preparation method of methoxybenzoic acid
A methoxybenzyl, acid-base technology, applied in the preparation of carboxylate, carboxylate, carboxynitrile and other directions, can solve the problems of large environmental pollution, large consumption of alkali, complicated operation, etc. The effect of less pollution, less alkali consumption and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 16.2g of 30% sodium methoxide and 0.1mol of o-chlorobenzonitrile into a 100ml autoclave, replace the air with nitrogen three times, seal the reaction kettle, raise the temperature to 100°C-120°C, the reaction pressure is about 0.3Mpa-0.5Mpa, and Insulate for 2 hours to 4 hours, HPLC analysis o-chlorobenzonitrile <0.5% is the end point, cool the reaction solution to less than 50°C, add 14.7g of 30% liquid alkali dropwise, heat up and distill to recover methanol, when the internal temperature reaches 100°C, Change to reflux, and keep the reflux reaction for 2 hours to 8 hours. When o-methoxybenzonitrile <0.5% is the end point, cool the reaction liquid, adjust the pH<4 with hydrochloric acid, and precipitate a large amount of white solid, filter, wash with water, and dry Dry to obtain o-methoxybenzoic acid.
Embodiment 2
[0043] Add 27.0 g of 30% sodium methoxide and 0.1 mol of o-chlorobenzonitrile to a 100 ml autoclave, replace the air with nitrogen three times, seal the reactor, raise the temperature to 80°C to 100°C, and the reaction pressure is about 0.2Mpa to 0.4Mpa, and Insulate for 2 hours to 4 hours, HPLC analysis o-chlorobenzonitrile <0.5% is the end point, cool the reaction solution to less than 50°C, add 14.7g of 30% liquid alkali dropwise, heat up and distill to recover methanol, when the internal temperature reaches 100°C, Change to reflux, and keep the reflux reaction for 2 hours to 8 hours. When o-methoxybenzonitrile <0.5% is the end point, cool the reaction liquid, adjust the pH<4 with hydrochloric acid, and precipitate a large amount of white solid, filter, wash with water, and dry Dry to obtain o-methoxybenzoic acid.
Embodiment 3
[0045]Add 32.4g of 30% sodium methoxide and 0.1mol of o-chlorobenzonitrile to a 200ml autoclave, replace the air with nitrogen three times, seal the reaction kettle, raise the temperature to 130°C to 150°C, the reaction pressure is about 0.8Mpa to 1.4Mpa, and Insulate for 2 hours to 4 hours, HPLC analysis o-chlorobenzonitrile <0.5% is the end point, cool the reaction solution to less than 50°C, add 14.7g of 30% liquid alkali dropwise, heat up and distill to recover methanol, when the internal temperature reaches 100°C, Change to reflux, and keep the reflux reaction for 2 hours to 8 hours. When o-methoxybenzonitrile <0.5% is the end point, cool the reaction liquid, adjust the pH<4 with hydrochloric acid, and precipitate a large amount of white solid, filter, wash with water, and dry Dry to obtain o-methoxybenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com