Application of wild horse chasing sesquiterpene parts in the preparation of anti-acute lung injury drugs
A technology for acute lung injury and sesquiterpene, which is applied to the application field of wild horse sesquiterpene parts in the preparation of anti-acute lung injury medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
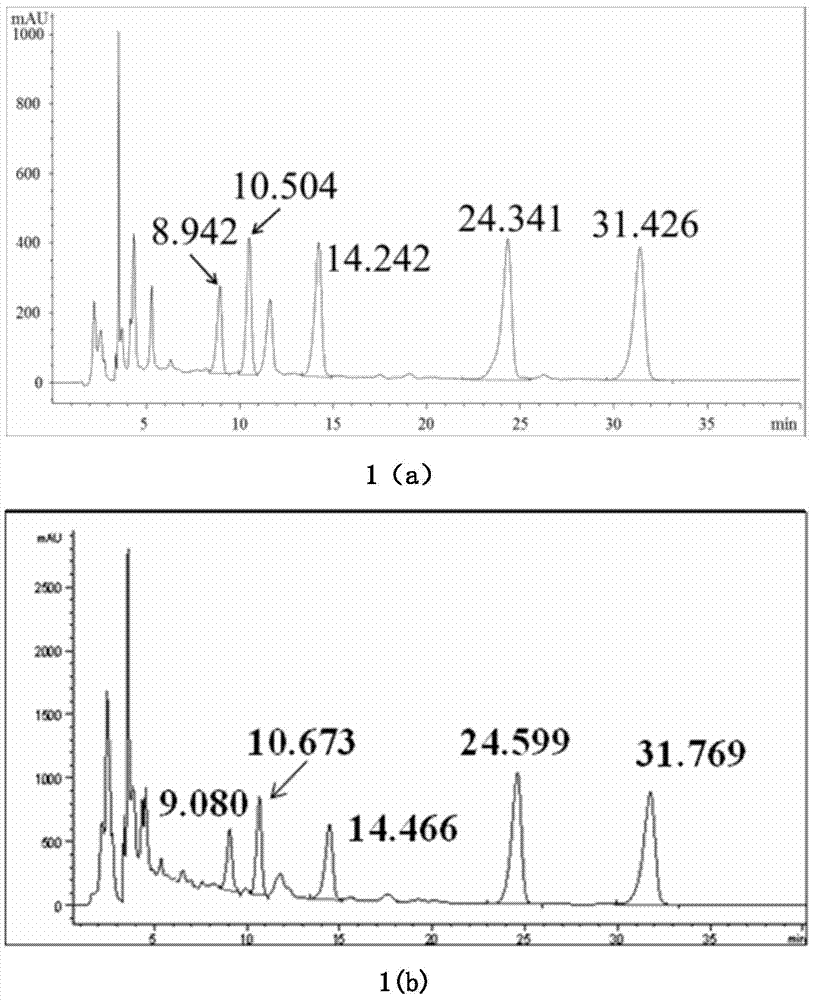
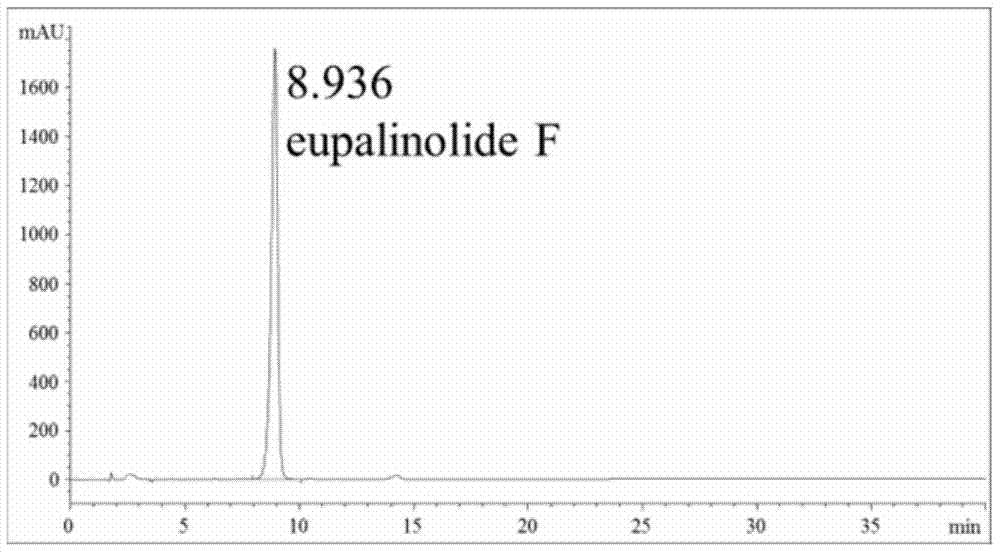
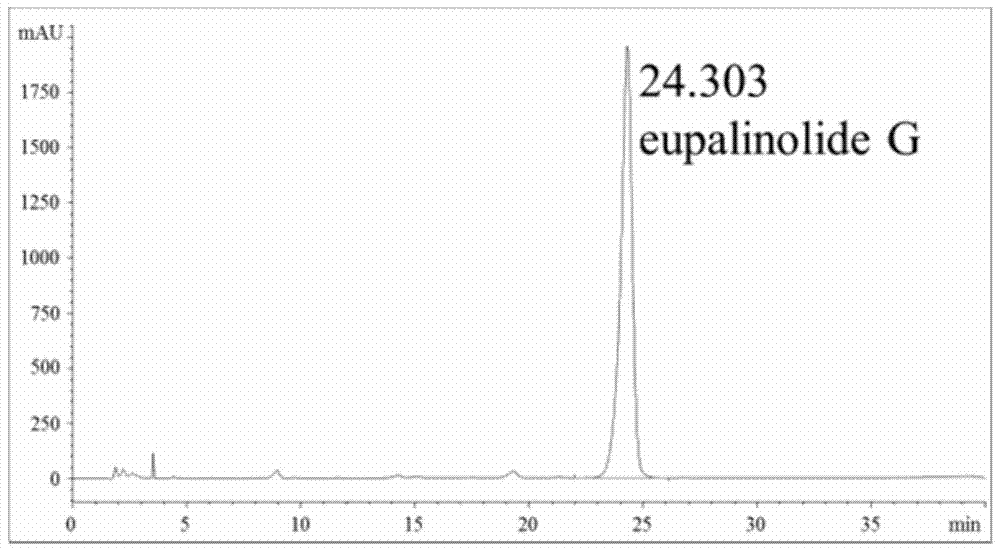
[0043] Preparation of Sesquiterpene Fraction from Yemazhui and Detection of Active Components
[0044] (1) Preparation of the sesquiterpene part of Yemazhui:
[0045] Sample 1: Weigh 300g of Yemazhui dried whole herb, cut into 2-3cm sections, add 10 times the volume of 80% ethanol aqueous solution, reflux extraction for 2 hours, repeat twice, recover the solvent under reduced pressure at 40°C to obtain Yemazhui Ethanol extract 45g; Dissolve this ethanol extract in about 90ml of water, absorb through 500ml of D101 macroporous adsorption resin, remove macromolecular compound with 5000ml water elution first, be 95% ethanol aqueous solution elution with 5000ml volume fraction behind, reduce The solvent was recovered under pressure to obtain 17g of macroporous resin; the obtained macroporous resin was adsorbed by 350ml of YMCODS-A reversed-phase silica gel filler particle size 60um reversed-phase silica gel column, eluted with 1750ml volume fraction of 50% methanol aqueous solution...
Embodiment 2
[0058] Anti-acute lung injury experiment of Yemazhui sesquiterpene fraction (EUP-SQT) in mice
[0059] 1. Experimental drugs and reagents: 0.5% sodium carboxymethylcellulose; lipopolysaccharide (LPS), Sigma-Aldrich, USA; dexamethasone acetate tablets, Zhejiang Xianju Pharmaceutical Co., Ltd.; polyclonal rabbit anti-human C3c complement, Shanghai Ruiqi Biotechnology Co., Ltd.; distilled water; normal saline; 20% urethane-normal saline; 4% formalin, H-E staining solution, 3,3'-diaminobenzidine (DAB) chromogenic solution, etc.
[0060] 2. Experimental mice: Balb / c mice, male, weighing 24-28 grams, provided by the Experimental Animal Center of Soochow University, experimental animal production license: XCYK (Su) 2002-0008. The experimental animals were raised in an environment with a temperature of 24±1° C. and a relative humidity of 40% to 80%, with free access to food and water. Before the experiment, they were adaptively fed for 7 days.
[0061] 3. Other materials: mouse tumo...
Embodiment 3
[0081] Comparative experiment on anti-acute lung injury effect of Yemazhui sesquiterpene part and Yemazhui ethanol extract
[0082] Preparation of Yemazhui ethanol extract: Weigh the dried whole herb of Yemazhui, cut it into sections of 2-3 cm, add 10 times the volume of 80% ethanol aqueous solution, reflux extraction for 2 hours, repeat twice, recover the solvent under reduced pressure, and obtain Yemazhui ethanol Extract (EtOH).
[0083] In this comparative experiment, the experimental reagents, experimental mice and experimental materials are the same as those in Example 2.
[0084] Experimental mice were randomly divided into 5 groups including blank group, model group, EtOH group of ethanol extract of Yemazhui, EUP-SQT group of sesquiterpene part of Yemazhui and positive group, 10 mice in each group. Yemazhui ethanol extract EtOH group was given EtOH at a dose of 30 mg·kg -1 ; Yemazhui sesquiterpene part SQT group was given EUP-SQT, the dose was 30mg·kg -1 ; The positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com