Application of acyl-coenzyme A oxidase as therapeutic target of diabetes
A technology of fatty acyl coenzyme and oxidase, which is applied in the fields of biochemistry and pharmacy, and can solve problems such as no reports of AOX inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] 1. Preparation of AOX inhibitor and its inhibitory effect in vitro
[0069] The present inventors prepared LH-919-CoA. Using LH-919 acid as raw material, high-purity LH-919-CoA was prepared, and its inhibitory effect on AOX was tested. The synthetic method of preparing fatty acyl-CoA from free fatty acid is carried out with reference to literature (Li D. et. al., J. Am. Chem. Soc., 2001, 121, 9034-9042).
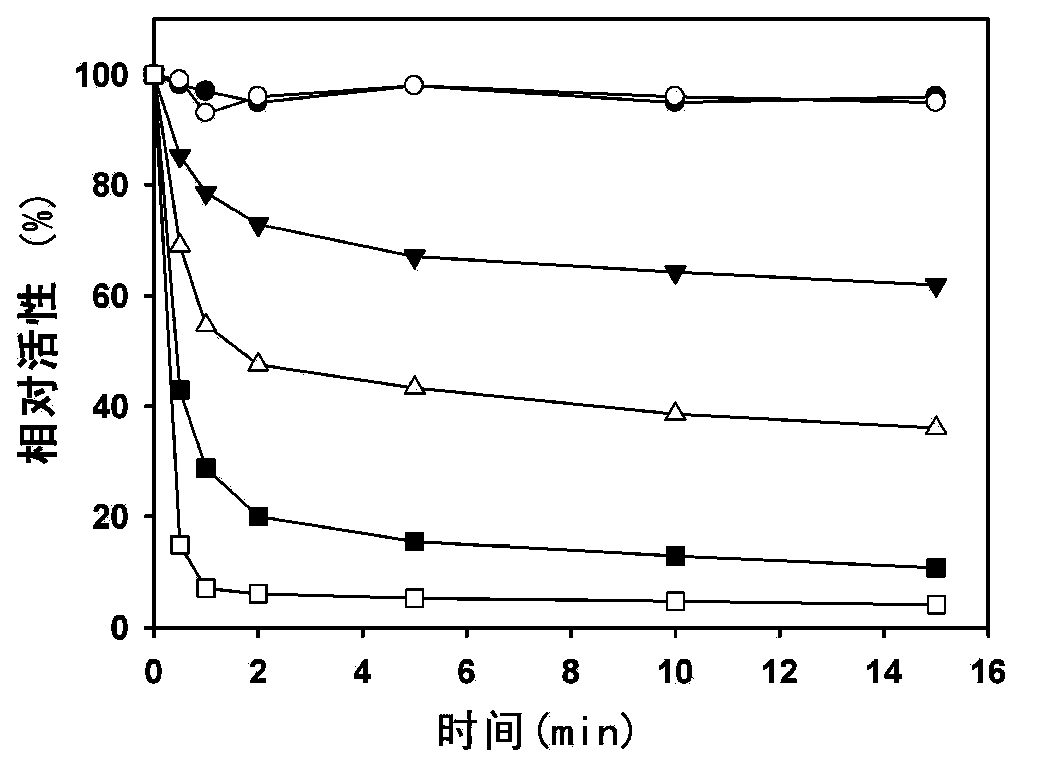
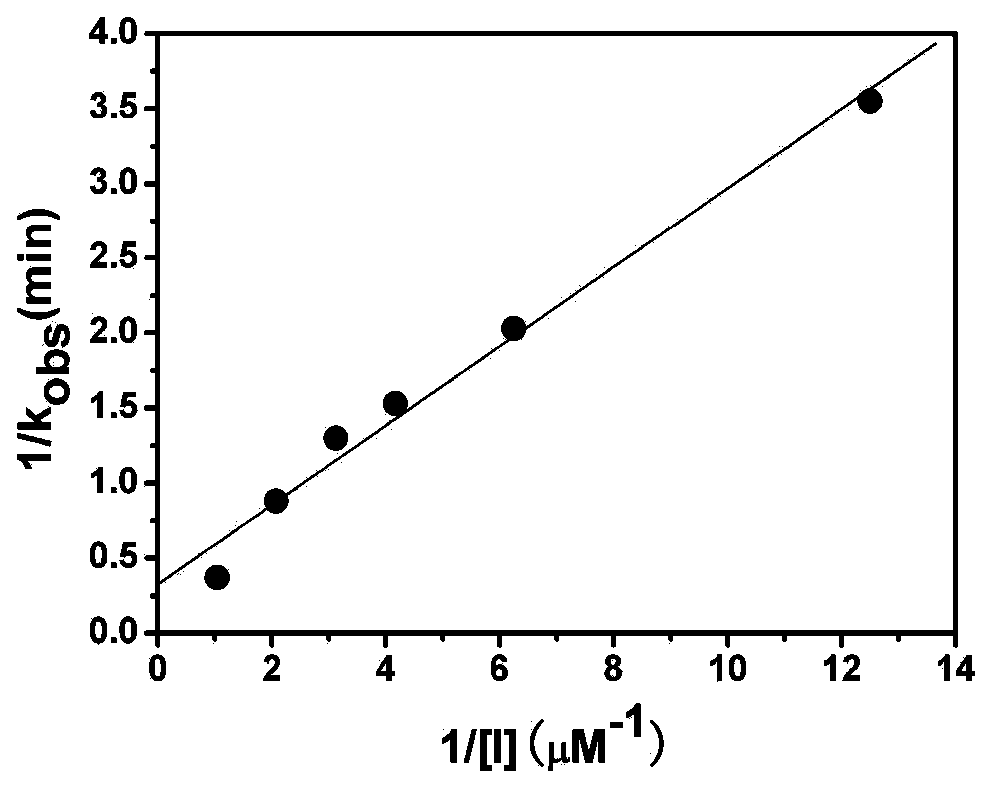
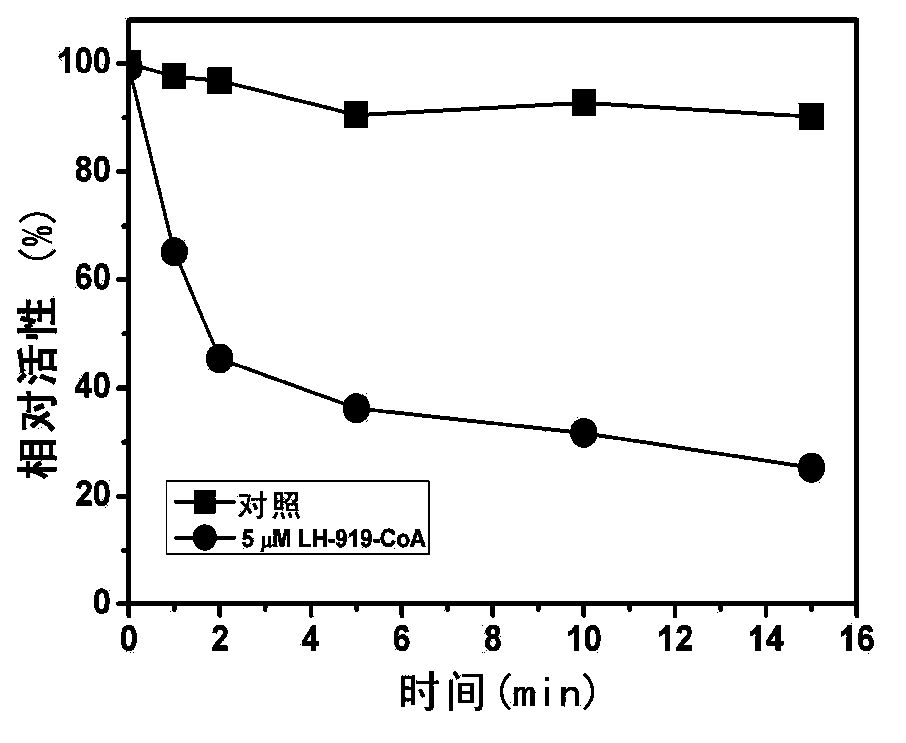
[0070] The inventors found for the first time that LH-919-CoA can significantly inhibit liver AOX activity in vitro. First, the recombinantly expressed and highly purified rat liver AOX was used as the target protein to determine the inhibitory effect of LH-919-CoA on AOX. The results showed that LH-919-CoA could rapidly inhibit the activity of AOX, and the inhibitory effect was irreversible. Free LH-919 acid had no inhibitory effect on AOX.
[0071] The rat liver intact peroxisome was used as the test object to test the inhibitory effect of LH-919-CoA on AOX in pe...
Embodiment 1
[0111] Embodiment 1, preparation of AOX inhibitor and in vitro inhibition experiment
[0112] (1) Preparation of AOX inhibitor LH-919-CoA
[0113] The preparation method of LH-919-CoA was carried out according to the literature (Li D. et. al., J. Am. Chem. Soc., 2001, 121, 9034-9042). Specific operation: LH-91920mg (Sigma, St.Louis, MO), add 5mL anhydrous tetrahydrofuran (tetrahydrofuran, THF) (Acros, Geel, Belgium), add 0.1mmol triethylamine (triethylamine) to the solution under nitrogen protection ) (Sigma, St.Louis, MO), after mixing and stirring for 10 minutes, 0.1 mmol isobutyl chloroformate (isobutyl chloroformate) (Acros, Geel, Belgium) was added dropwise to the solution at 0° C., and stirred at room temperature for 1 h. Finally, dissolve 50 mg of coenzyme A sodium salt (Coenzyme A) (USB, Cleveland, OH) in distilled water, add 1 mol / L NaOH to adjust the pH to 8.0, add dropwise to the reaction solution under nitrogen protection, and continue stirring for 20 minutes. Sl...
Embodiment 2
[0131] Example 2. Analysis of the effect of AOX inhibitor precursors on inhibiting AOX in rats and mice
[0132] (1) Experimental determination of the transformation of LH-919 into LH-919-CoA in animals
[0133] Six SPF Wistar rats, male, weighing 220-250 g, were fasted for 12 hours, and the six rats were divided into two groups, the control group and the drug group, with three rats in each group. The control group was given 0.2 mL of olive oil by gavage. In the drug group, LH-919 was dissolved in the corresponding volume of olive oil, 100 μg / kg of LH-919 was intragastrically administered, and all the rats were sacrificed 3 hours later, and the livers were quickly dissected and removed, and immediately placed in liquid nitrogen for preservation.
[0134] The determination of fatty acyl-CoA in subcellular unit contents in liver cells was carried out by reference (Melde K. et. al., Biochem. J., 1991, 274, 395-400). The operation steps are as follows: Accurately weigh 0.3 g of l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com