Small molecular antibody affinity peptide and application thereof
A small molecule antibody and affinity peptide technology, applied in the field of molecular biology, can solve the problems of protein A instability, easy shedding of ligands, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2I
[0040] Embodiment 2 IgG affinity property determination
[0041] 1) Prepare 10mmol / L Hepes buffer, pH 7.4, NaCl content 150mmol / L.
[0042] 2) Use the liquid prepared in 1) as the mobile phase, and run the BiacoreT200 analyzer.
[0043] 3) Insert the detection chip CM5 and set the flow rate to 30 μL / min.
[0044] 4) Inject 100 μL each of EDC and NHS in sequence to activate the chip.
[0045] 5) Dissolve IgG in sodium acetate buffer, pH 4.5, IgG 100μg / mL.
[0046] 6) Inject 100 μL of the IgG solution in 5), and immobilize IgG on the surface of the chip.
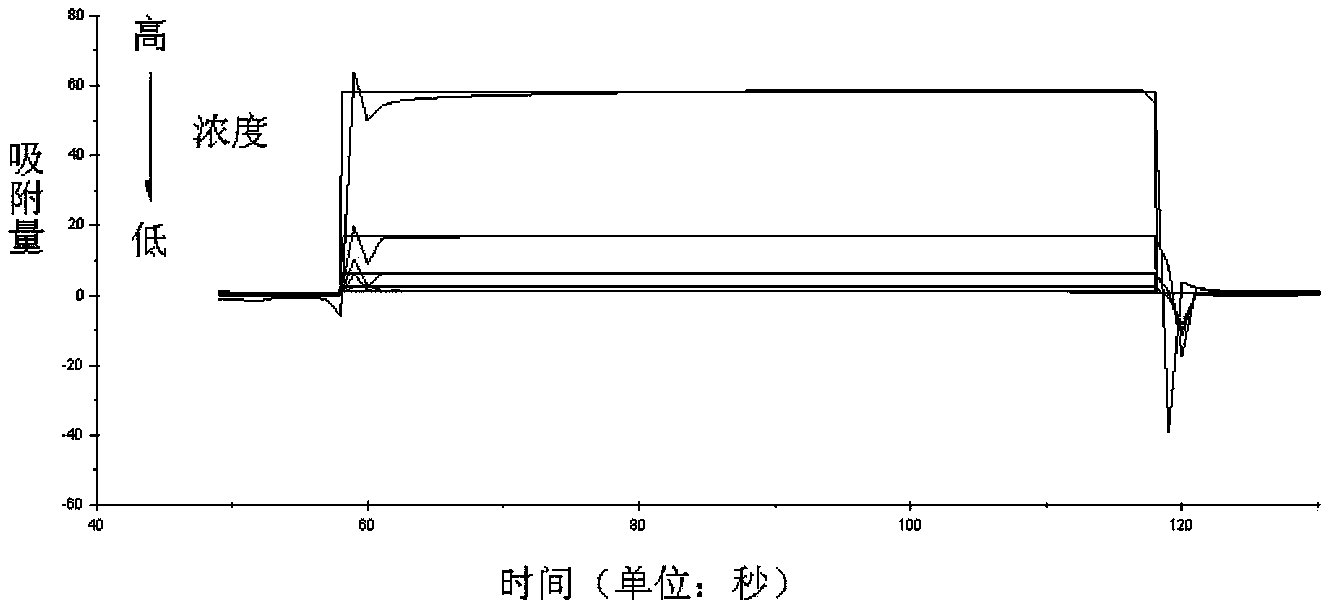
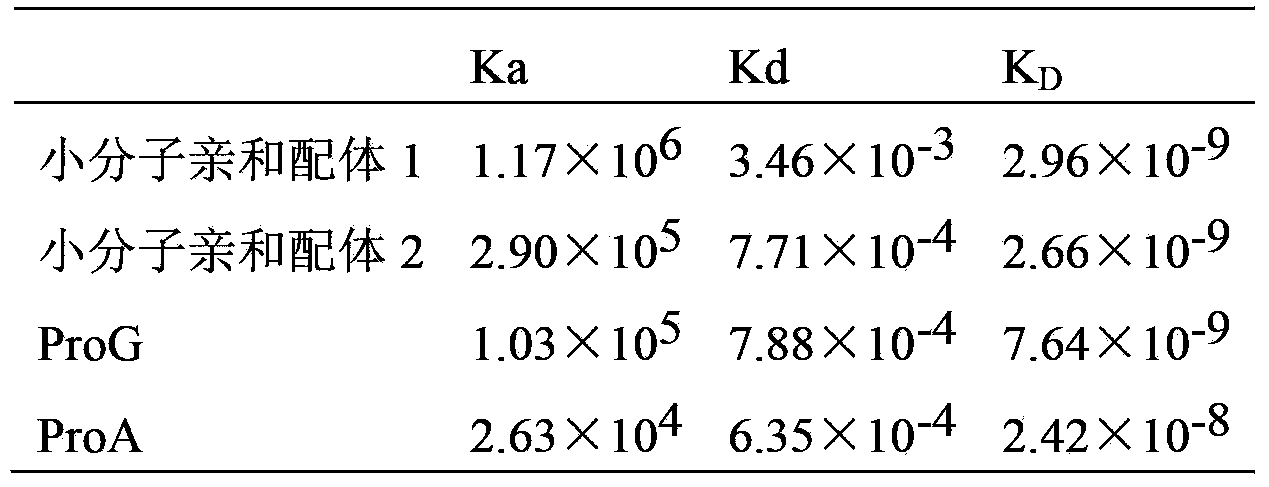
[0047] 7) Small molecule antibody affinity peptide 1 (amino acid sequence shown in SEQ ID No.11), small molecule antibody affinity peptide 2 (amino acid sequence shown in SEQ ID No.34) and natural ligand (ProG and ProA), dissolved in 1) to prepare liquid, content 100μg / mL.
[0048] 8) Sequentially inject different concentrations of peptides and natural ligands (concentrations from high to low are 500 μm, 250 μm, 125 μm, 6...
Embodiment 3
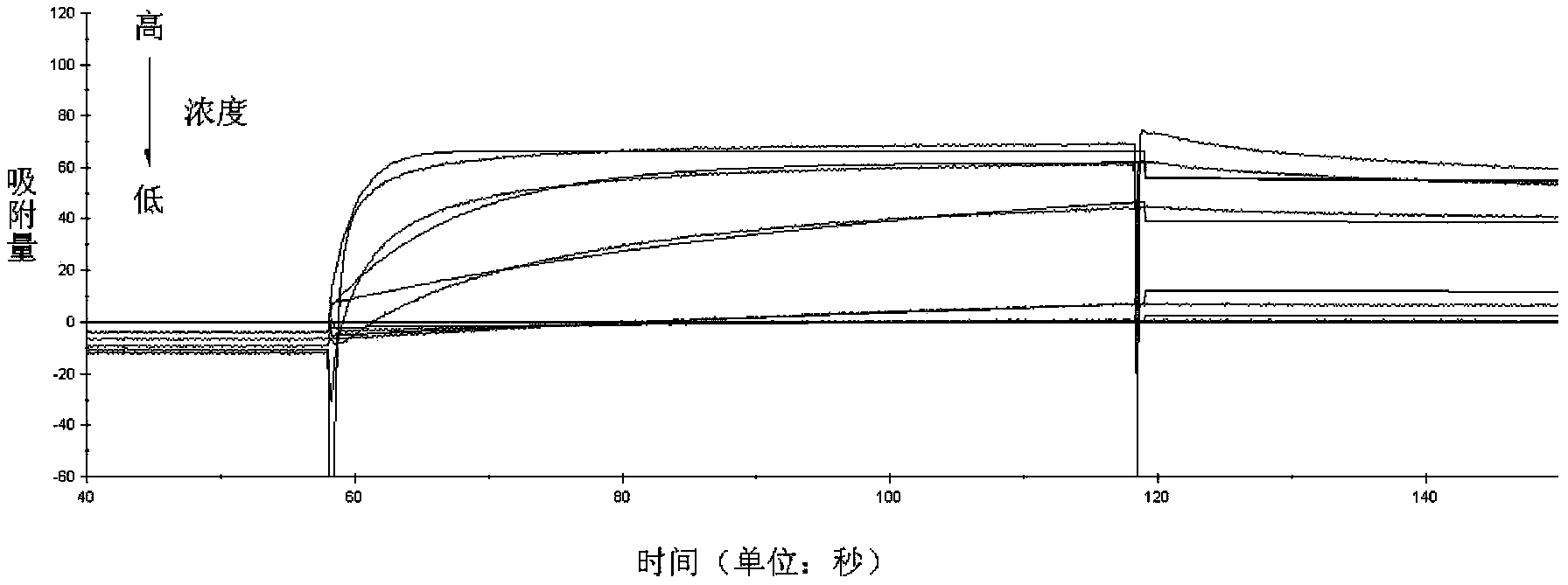
[0053] Example 3 Comparison of Adsorption Properties of Small Molecule Antibody Affinity Peptides with IgG and HAS
[0054] 1) Prepare 10mmol / L Hepes buffer, pH 7.4, NaCl content 150mmol / L.
[0055] 2) Use the liquid prepared in 1) as the mobile phase, and run the BiacoreT200 analyzer.
[0056] 3) Insert the detection chip CM5 and set the flow rate to 30 μL / min.
[0057] 4) Inject 100 μL each of EDC and NHS in sequence to activate the chip.
[0058] 5) Dissolve IgG in sodium acetate buffer, pH 4.5, IgG 100μg / mL. Also dissolve HSA in sodium acetate buffer, pH 4.5, HSA 100 μg / mL.
[0059] 6) Inject 100 μL of the IgG solution in 5), and immobilize IgG on the surface of the chip.
[0060] 7) Inject 100 μL of the HSA solution in 5), and immobilize HSA on the surface of another chip.
[0061] 8) Dissolve small molecule antibody affinity peptide 3 (amino acid sequence shown in SEQ ID No.1) and small molecule antibody affinity peptide 4 (amino acid sequence shown in SEQ ID No.4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com