Methods of decomposing middle- and low-grade phosphate ore with double acids to produce polyphosphoric acid
A technology of polyphosphoric acid and phosphate rock, applied in the field of hydrochloric acid wet-process phosphoric acid and its purification, can solve the problems of occupation, difficult phosphoric acid, low phosphoric acid concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
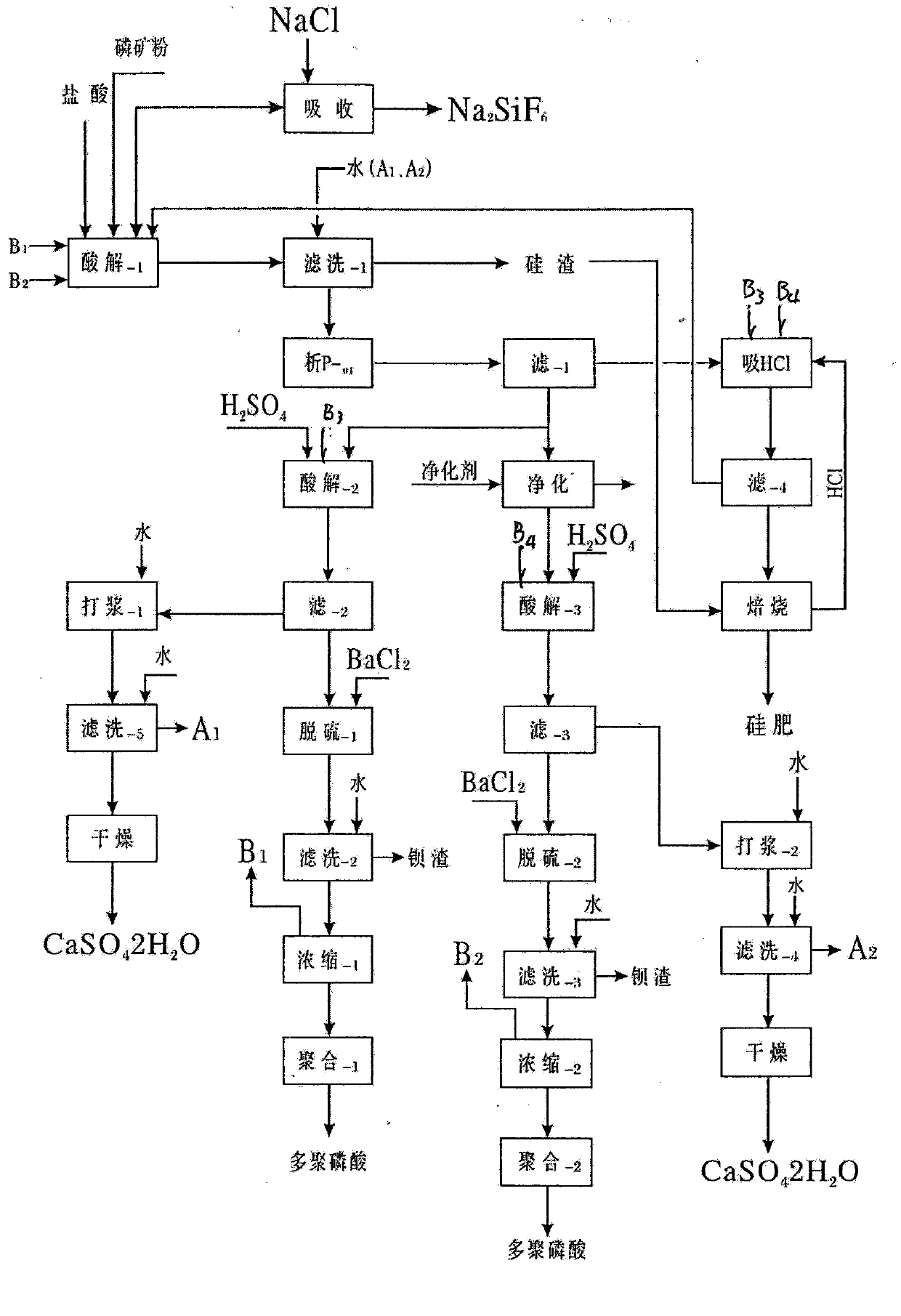
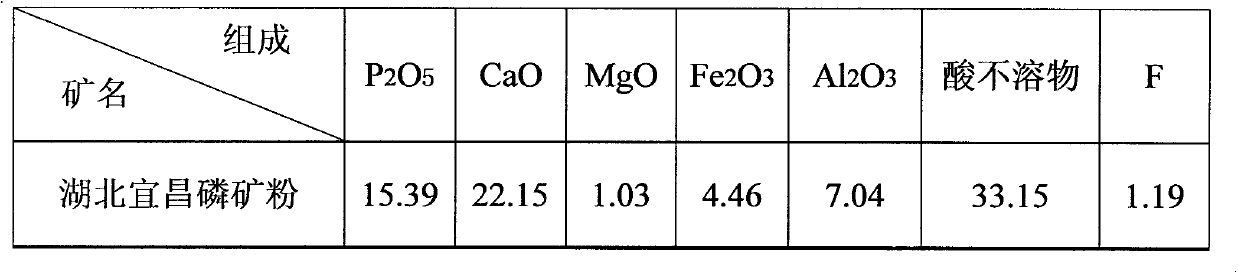
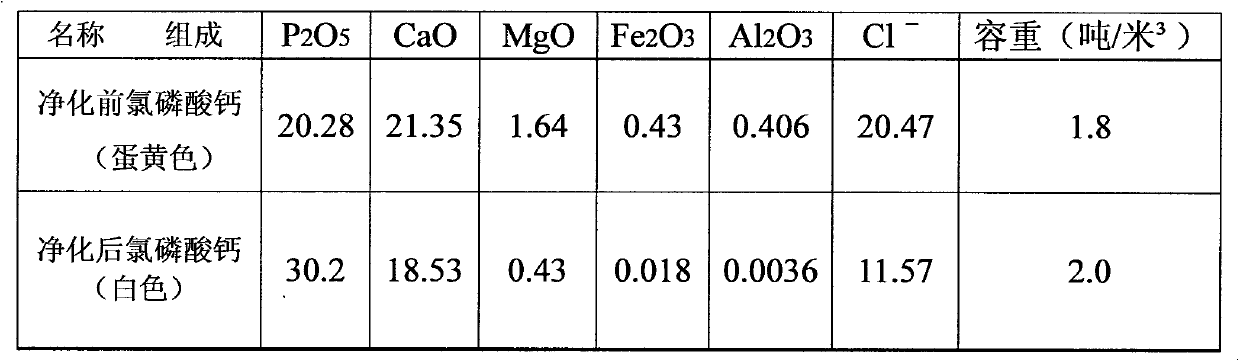
[0015] from P 2 o 5 =6.3% to 36% of all phosphate rocks can be smoothly processed into polyphosphoric acid, among which the phosphate rocks in the above table 1 are the most. In the cycle test, the general cycle is more than 4 times, and the P in the process parameters 2 o 5 The content in each process material has stabilized, and the 5th cycle is described below: add 300mlHCl=30% industrial hydrochloric acid in a 5000ml beaker, then add the hydrochloric acid and sulfuric acid returned by the calcium chloride and silicon slag roasting of the previous cycle Decompose P -01 Heat and stir the released hydrochloric acid and the hydrochloric acid released from the concentration of phosphoric acid, and slowly add 1000g of Yichang phosphate rock in Table 1 at 60°C, and stir at 80-85°C for 10 minutes after throwing in, suction and filter while it is hot, and use gypsum beating solution and washing water to rinse to obtain 2041g of decomposition solution and 414g of wet silicon slag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com