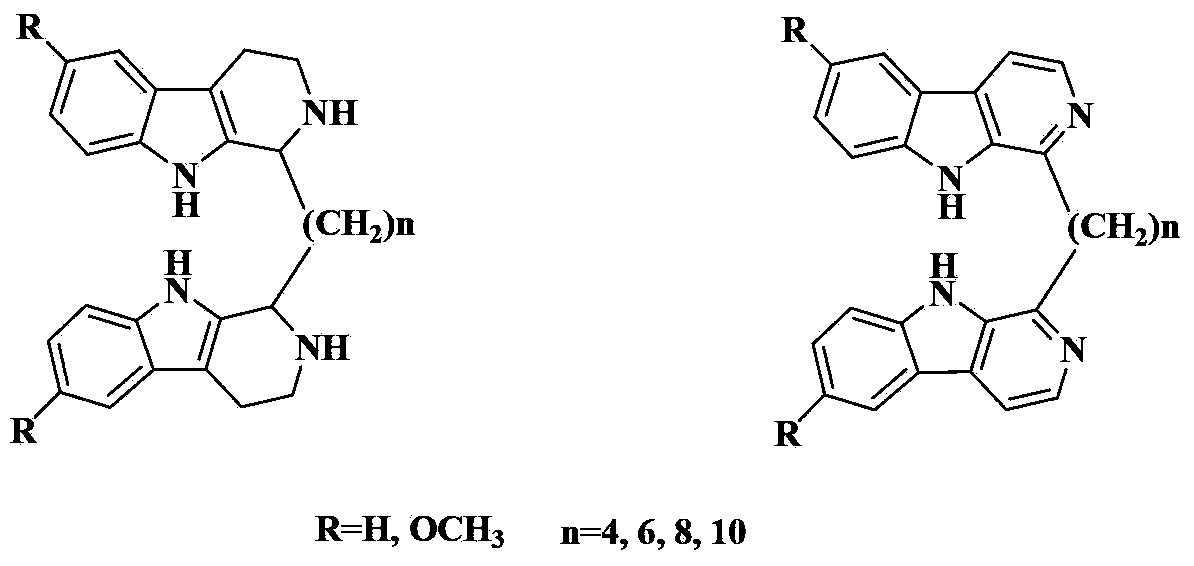
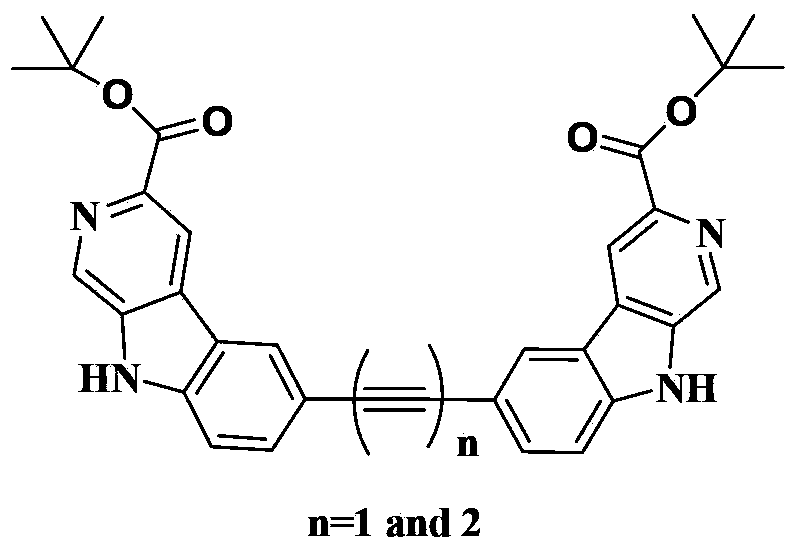
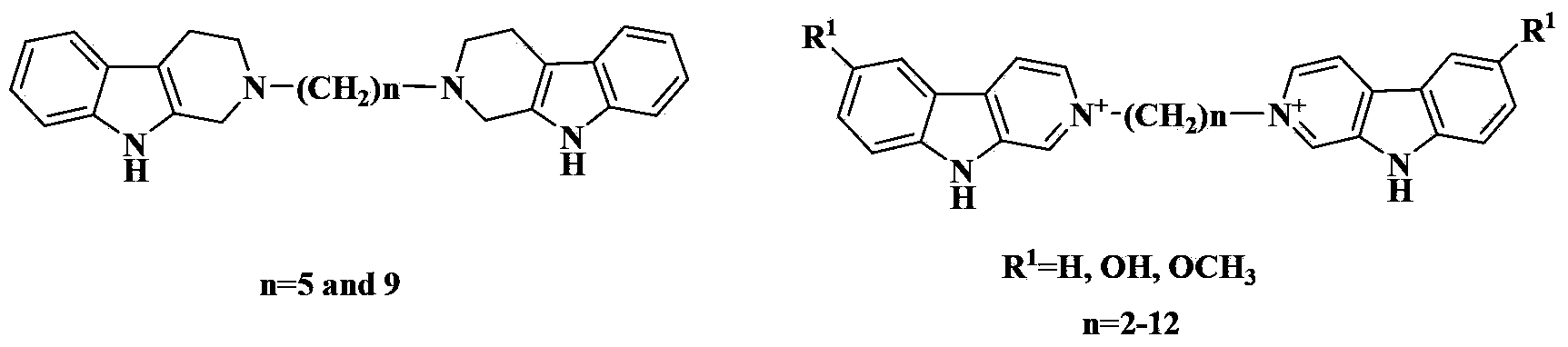
3-Position diamine beta-carboline alkali compound, and preparation method, medicinal composition and use thereof
A technology of compounds and medicinal salts, applied in the application field of 3-position diamine β-carboline base compounds, in the preparation of anti-tumor drugs, which can solve the problems of not disclosing anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0146]
[0147] 1. Preparation of 1-substituted-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid 2a-h:
[0148] 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid (2a):
[0149] Mix L-tryptophan (102g, 0.5mol) and NaOH (20g, 0.5mol), add 800ml of water, and stir magnetically. After the solution is clarified, add 37-40% formaldehyde (37.5ml, 0.54mol), and rinse the measuring device with about 100ml of water. Reaction at room temperature for 3.5h, then changed to heating under reflux for 2.5h. After the reaction is complete, cool it down, adjust the pH to about 6 with concentrated HCl, and put it in the refrigerator overnight. Filter, rinse the solid with water several times, and finally bring the water once with a small amount of acetone. After drying, a white solid was obtained with a yield of 98.5%.
[0150] 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2b):
[0151] L-tryptophan (0.4mol) and 600mlH 2 O mixed, then added 7.5ml H 2 SO 4 (0.5mol / L), after ...
preparation example 2
[0258]
[0259] General preparation of intermediates 9a-e:
[0260] Add ethyl β-carboline-3-carboxylate 8a or 8b (10mmol) and 30ml DMF to a 100ml round bottom flask, stir at room temperature until the solution is clear, then add 60% NaH (0.8g, 20mmol), and stir at room temperature for 15 minutes , and then added haloalkane (30mmol), stirred at room temperature, TLC tracking detection. After the reaction is complete, pour the reaction solution into ice water, extract with ethyl acetate (100ml×3), combine the organic phases, wash with water, wash with saturated brine, dry, filter, and concentrate to dryness under reduced pressure, silica gel column chromatography, ethyl acetate: Petroleum ether = 1:2 elution, the target product was collected and concentrated to dryness under reduced pressure to obtain a white solid.
[0261] Ethyl 9-isopropyl-β-carboline-3-carboxylate (9a)
[0262] Starting from ethyl β-carboline-3-carboxylate and 2-bromopropane, a white solid (1.8g, 64%) w...
Embodiment 1
[0298]
[0299] General Preparation Process of 3-Aminoalkyl Chain Linked Bis-β-Carboline Base 1-39
[0300] Add the corresponding β-carboline-3-carbaldehyde (2mmol) and anhydrous methanol (30ml) in a 100ml round bottom flask, stir at room temperature for 10 minutes, then add the corresponding diamine (1mmol), and the reaction mixture is heated to reflux 2 hours. Reflux was stopped, methanol was distilled off under reduced pressure, and absolute ethanol was taken with water twice. The reaction residue was dissolved in anhydrous methanol (30ml), then sodium borohydride (5mmol) was added, the reaction was stirred at room temperature for 4-6 hours, followed by TLC detection. After completion of the reaction, carefully add concentrated hydrochloric acid (10ml) to the reaction solution, stir at room temperature for 15 minutes, then basify with concentrated sodium hydroxide solution, extract twice with dichloromethane (100ml), combine the organic phases, wash with water, and wash...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com