Preparation method of recombinant human albumin-uricase fusion protein
A uricase and protein technology, applied in the field of biotechnology genetic engineering, can solve the problems of restricting wide application and low microbial enzyme production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example Escherichia coli expression vector and the construction of expression strain
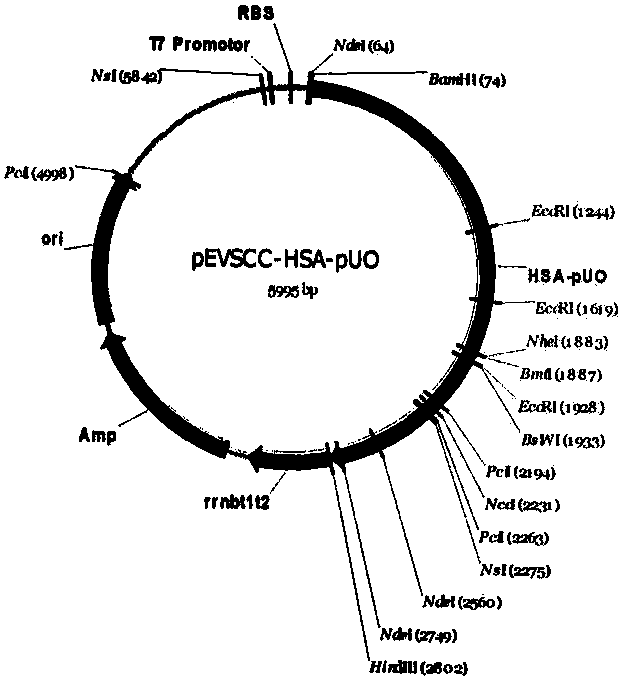
[0034] The designed coding genes of human serum albumin HSA and porcine uricase (UrATe oxidase of swine, pUO) were synthesized by Shanghai Handsome Biotechnology Company, and BamH I (5' end) and Nhe I were designed at both ends of the HSA gene fragment (3' end) restriction endonuclease sites, Nhe I (5' end) and Hind III (3' end) restriction endonuclease sites were designed at both ends of the pUO gene fragment respectively. Shanghai Handsome Biotechnology Co., Ltd. synthesized the gene fragment and cloned it into the pMD18T vector. After the sequence was correct, the E. coli containing the recombinant plasmid was sent back to the company.
[0035] Escherichia coli strains containing the coding genes of HSA and pUO were respectively inoculated into LB medium containing ampicillin, cultured overnight at 37°C with shaking, and the recombinant plasmid was extracted the next day according ...
Embodiment 2
[0038] Fermentation, purification and preparation of embodiment two engineering bacteria
[0039] Fermentation takes the strains of the basic seed bank for production, and after opening, mark 2 to 3 plates of seed medium (LB+Amp), pick 8 to 10 typical colonies on the plate after culture, store them after culture respectively, and then carry out recombinant uric acid respectively The determination of enzyme protein expression was repeated three times, and the one with the highest expression was selected for production and preparation of seed liquid. Pick a single bacterium and inoculate it in a test tube of LB liquid medium, culture and shake in a shaker at 32°C for 12 hours. Put the bacteria in the test tube into the shake flask with the inoculum amount of 1:100, and cultivate in the remote bed at 32°C until OD600=3, that is, the fermenter can be inoculated at a ratio of 10%. The fermentation medium is a modified LB medium, prepared with distilled water, which does not contai...
Embodiment 3
[0043] The mensuration of embodiment three enzyme activity
[0044] Referring to the method of Koyama et al. (1996), at 37°C, take 120 μmol / L uric acid solution dissolved in 3ml pH7.5 boric acid buffer solution, add an appropriate amount of uricase solution to a 1cm quartz cuvette, mix well, and place in UV -1601PC ultraviolet spectrophotometer, react accurately for 5 minutes, use boric acid buffer as blank, and measure the decrease of absorbance at 293nm. The activity unit is defined as the amount of enzyme required to catalyze 1 μmol of uric acid into allantoin within 1 min under the conditions. Its calculation formula is:
[0045] U / ml=(ΔA×VT×df) / (12.6×VE), in the formula: U=number of uricase activity units per ml; ΔA=optical density drop value at 293nm wavelength per minute; VT=total volume of reaction solution (ml); df=dilution factor; 12.6 is the micromolar extinction coefficient of uric acid at a wavelength of 293nm; VE=enzyme solution volume (ml).
[0046] Relative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com