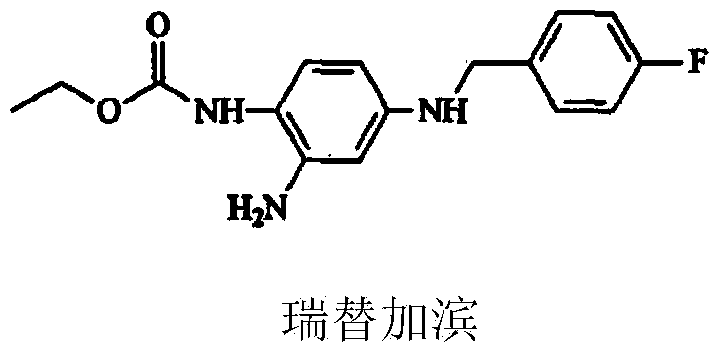
Retigabine micro pellet capsule and preparation method thereof
A technology of retigabine and retigabine pellets, applied in capsule delivery, pharmaceutical formulation, drug delivery, etc., can solve the problems of slow dissolution, drug waste, drug side effects, etc., reduce taste, increase stability, and reduce particle size The effect of uniform diameter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 prepares retigabine micropill capsule
[0073] Retigabine 500g
[0074] Microcrystalline Cellulose Core 222g
[0075] Hypromellose E5 52g
[0076] Croscarmellose Sodium 16g
[0077] Opadry 03F 24g
[0078] Crushing of the raw material drug: the retigabine raw material was pulverized with a GTM-50 jet mill until its D90=6 μm; drug-loaded layer suspension preparation: measure 1100ml of water, dissolve hypromellose in it, and then add The crushed retigabine raw material and croscarmellose sodium in the prescribed amount were stirred until uniformly dispersed, and passed through an 80-mesh sieve to obtain a suspension of the drug-loaded layer; The parameters are as follows: air volume 100m 3 / h, liquid supply speed 8g / min, atomization pressure 1.7bar, air inlet temperature 50°C, material temperature 40°C, spacer height 10cm; fluidized bed drying for 20min after the drug application, the parameters are as follows: air volume 90m 3 / h, liquid supply rate 0g...
Embodiment 2
[0085] Embodiment 2 prepares retigabine micropill capsule
[0086] Retigabine 500g
[0087] Sucrose core 100g
[0088] Hydroxypropyl Cellulose EF 100g
[0089] Low-substituted hydroxypropyl cellulose 32g
[0090] Opadry II 40g
[0091] Grinding of raw materials: Grinding the retigabine raw material with a KEQ-2L ball mill until its D90=20 μm; preparation of drug-loaded layer suspension: measure 2000ml of water, dissolve hydroxypropyl cellulose in it, and then add the prescribed amount The pulverized retigabine raw material and croscarmellose sodium were stirred until uniformly dispersed, and passed through an 80-mesh sieve to obtain a drug-loaded layer suspension; LZW-300 centrifugal granulation coating machine was used for drug application, and the parameters As follows: air volume 60m 3 / h, liquid supply rate 10g / min, atomization pressure 1.8bar, air inlet temperature 55°C, material temperature 40°C, turntable speed 20r / min; oven dry at 60°C for 2h after drug applicatio...
Embodiment 3
[0092] Embodiment 3 prepares retigabine micropill capsule
[0093] Retigabine 500g
[0094] Lactose core 400g
[0095] Povidone K30 50g
[0096] Sodium carboxymethyl starch 8g
[0098] Opadry 03K 48g
[0099] Grinding of the raw material: Grinding the retigabine raw material with a jet mill until its D90=11 μm; preparation of the adhesive: taking 1000ml of water, dissolving povidone K30 in it, stirring until uniformly dispersed, passing through an 80-mesh sieve That is ready; mix: mix the retigabine raw material and carboxymethyl starch sodium after the prescription amount is pulverized evenly; apply the medicine with LZW-300 centrifugal granulation coating machine, the parameters are as follows: air volume 50m 3 / h, liquid supply speed 10g / min, atomization pressure 1.6bar, air inlet temperature 55°C, material temperature 40°C, turntable speed 20r / min, feed hopper speed 6r / min; fluidized bed drying for 20min after drug application; Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com