An organic small molecule material based on spirothioxanthene and an organic photoelectric device using the material as a light-emitting layer
A technology of optoelectronic devices and small molecules, applied in the direction of electric solid devices, electrical components, luminescent materials, etc., can solve the problems of organic small molecule materials that are rarely reported, and achieve the effect of improving carrier transport characteristics and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
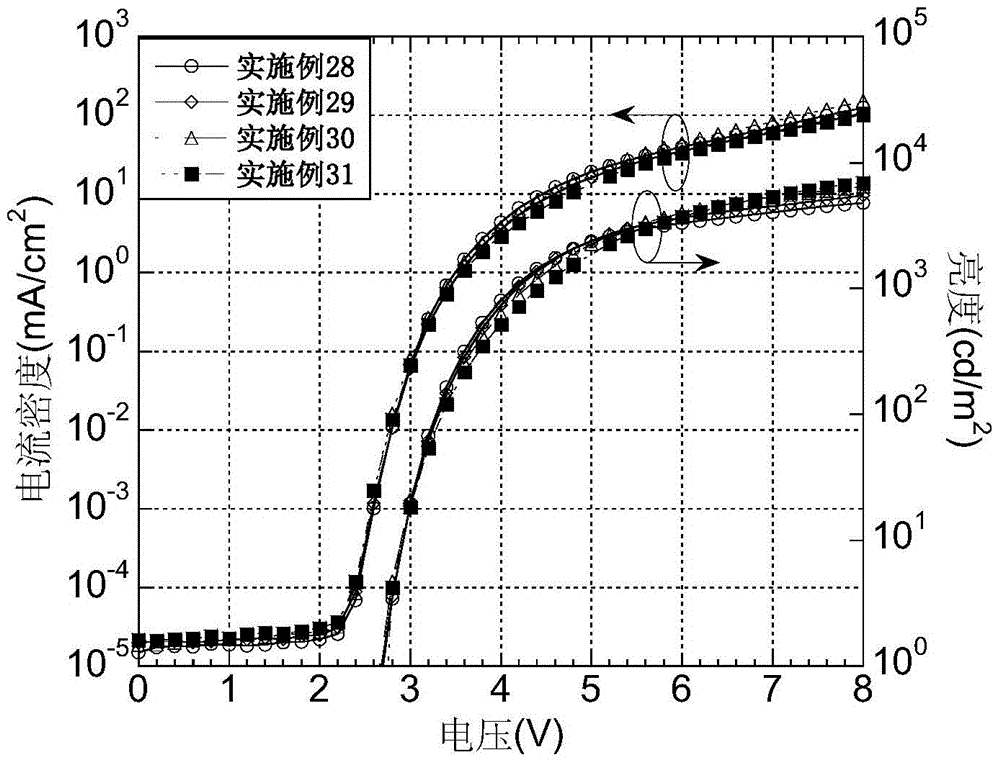
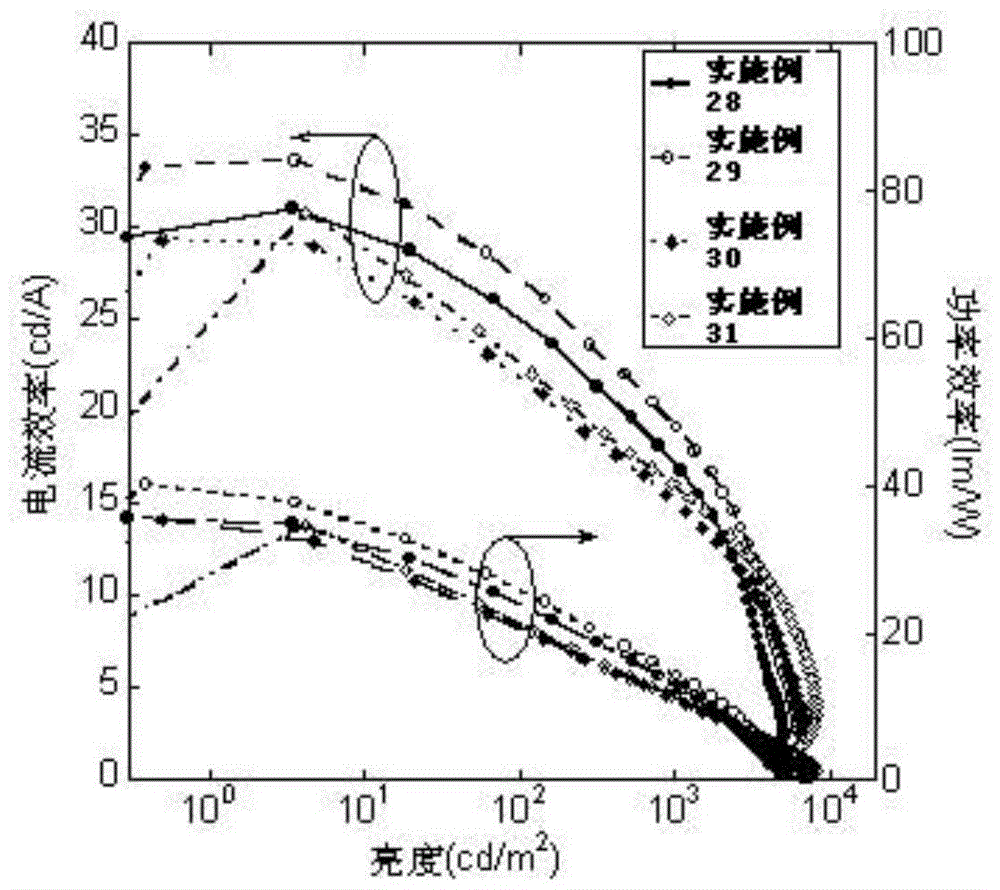
Examples
Embodiment 1
[0033] The preparation of present embodiment intermediate 1 and 2:
[0034] Synthesis of 2-bromodiphenylsulfide: o-bromoiodobenzene (50mmol, 14.3g), diphenyldisulfide (16.625mmol, 3.40g), Cu 2 S(1.194g), Fe(1.05g), K 2 CO 3 (8.625g) was dissolved in 125ml of DMSO, heated to 120°C overnight to obtain 2-bromodiphenylsulfide (3.84g), yield 93%. 1H NMR: 7.55-7.59 (m, 1H), 7.36-7.48 (m, 5H), 7.13-7.18 (m, 1H), 7.01-7.07 (m, 1H), 6.92-6.96 (m, 1H). The reaction equation is as follows:
[0035]
[0036] Synthesis of Intermediates 1 and 2: Dissolve 2-bromodiphenylsulfide (11mmol, 2.93g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, and keep warm for 1 hour , Dissolve 4,4'-dibromobenzophenone (10mmol, 3.38g) in 30ml THF, add to react overnight, extract with dichloromethane, and pass through a silica gel column. White solid a (2.35g) was obtained with a yield of 53%; the white solid a (4.46mmol, 2.35g) was directly added to glacial acetic acid, heated...
Embodiment 2
[0039] In this embodiment, the preparation of a spirothioxanthene-based organic small molecule material having the structural formula P5:
[0040] Intermediate 2 (1.5mmol, 0.81g) obtained in Example 1, carbazole (3.3mmol, 0.57g), CuI (0.23g), K2CO3 (0.55g), C18O6 (0.1g), were dissolved in 1,3 -Dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU), heated to 180°C overnight, extracted with dichloromethane, passed through a silica gel column to obtain 0.78g P5, yield 90% . 1H NMR: 8.33-8.34 (d, 2H), 8.14-8.16 (d, 4H), 7.65-7.67 (d, 4H), 7.56-7.58 (d, 4H), 5.49-5.51 (d, 4H), 7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
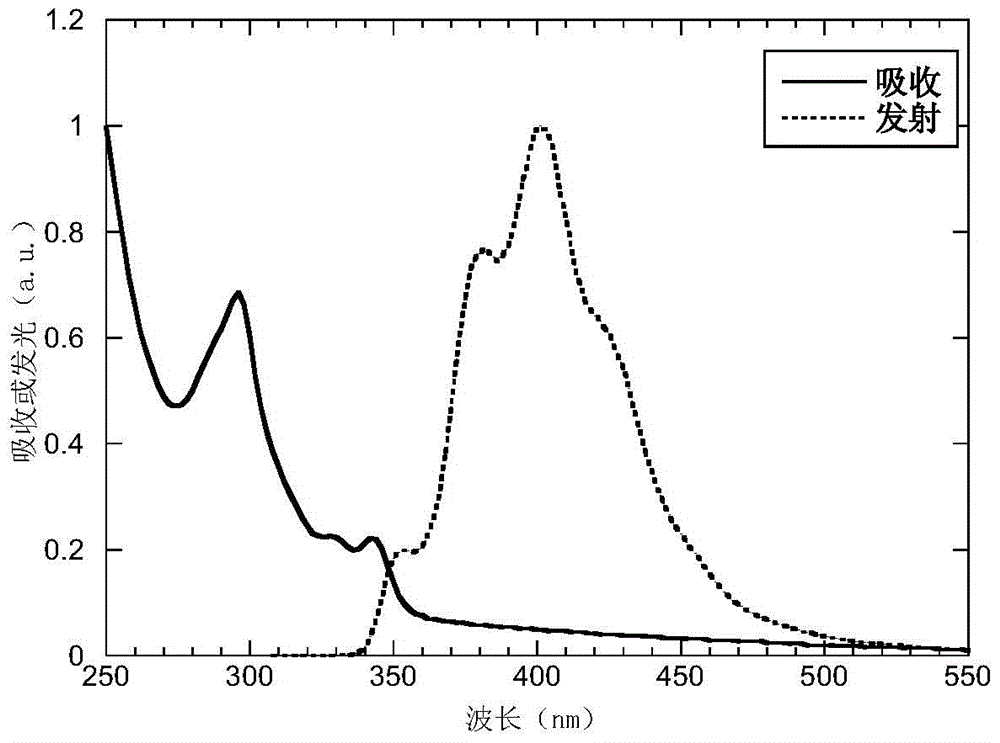
[0041] The absorption and emission spectrograms of the P5 of the present embodiment in the thin film state are as follows figure 1 As shown, from the absorption edge wavelength λ=358nm of the film, according to the formula optical band gap Eg opt =1240 / λ learned, Eg opt = 3.46eV.
Embodiment 3
[0043] In this embodiment, the preparation of a spirothioxanthene-based organic small molecule material having the structural formula P6:
[0044]Intermediate 1 (1.5mmol, 0.81g) obtained in Example 1, carbazole (3.3ml, 0.57g), CuI (0.23g), K2CO3 (0.55g), C18O6 (0.1g), were dissolved in DMPU, Heated at 180°C overnight, extracted with dichloromethane, and passed through the column to obtain 0.83g of P6. The yield was 82%. 1H NMR: 8.85-8.84 (d, 2H), 8.14-8.16 (d, 4H), 7.65-7.67 (d, 4H), 7.56-7.58 (d, 4H), 5.49-5.51 (d, 4H), 7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com