Thrombin-inhibited polypeptide and preparation method and application thereof
A technology for inhibiting peptides and thrombin, applied in the field of medicine, can solve the problem of no thrombin inhibitor coming out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Chemical Synthesis of Peptides
[0013] Polypeptide 2 was prepared by Fmoc-protected solid-phase synthesis technique. The synthesis reaction was carried out from C-terminal to N-terminal according to the sequence of polypeptide 2, and there were free amino groups on Rink medium (available from Advanced Chem Tech). During each ligation step, the amino acid residues are activated, and the activation mixture contains 4 times as many HBTU, HOBt, DIEA and Fmoc-amino acids as there are free amino groups on the medium. After each amino acid linking reaction, a mixture of pyridine / acetic acid / N-methylimidazole (3:2:0.5) was used to block unlinked free amino groups for 10 minutes. After each amino acid linking reaction and before the next amino acid linking, the Fmoc-group on the medium should be removed, and the Fmoc-group should be removed using dimethylformamide containing 20% piperidine, which takes 15 minutes. Finally, after all amino acid residues were linked sequentia...
Embodiment 2
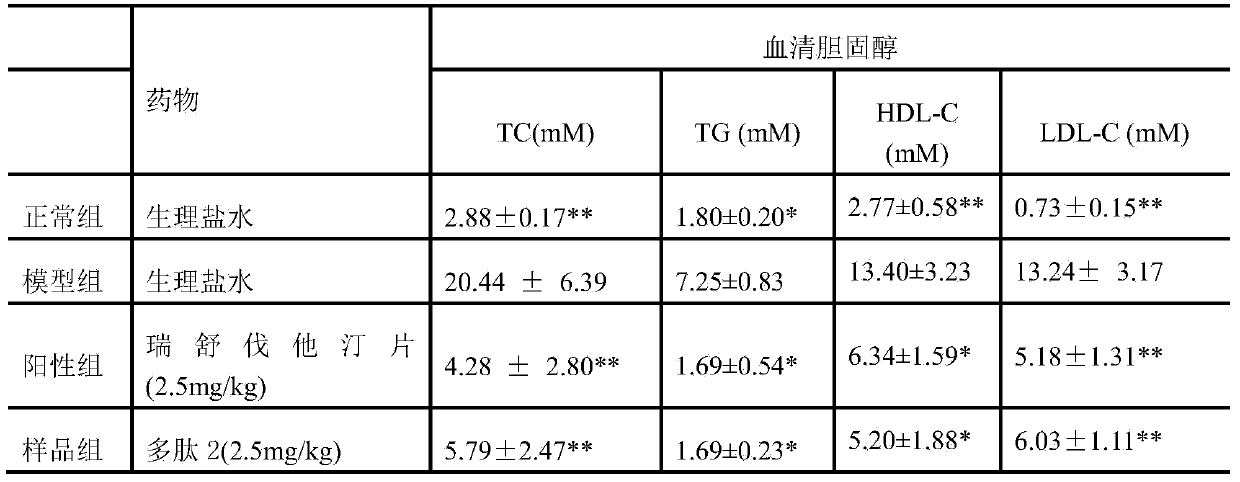
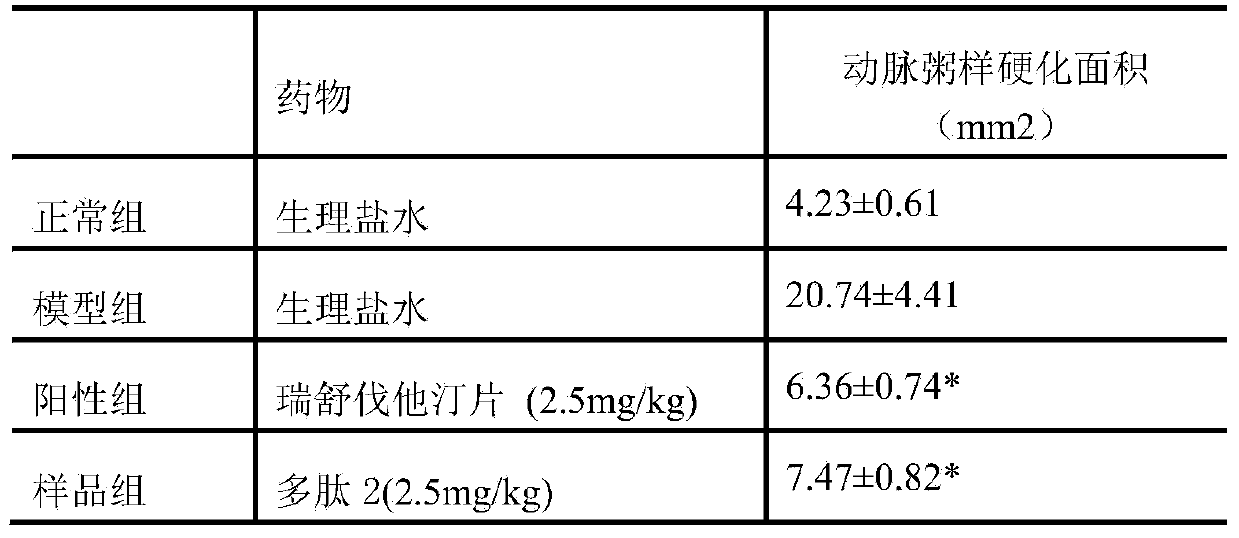
[0016] Effect of thrombin inhibitory polypeptide 2 on rabbit model of atherosclerosis
[0017] Animal model of rabbit atherosclerosis model was established. 40 male New Zealand white rabbits were randomly divided into 4 groups, respectively: Group A was the blank control group: fed with common feed; Group B was the model control group: model-building + high-fat feed fed; Model + high-fat feed + thrombin inhibitory polypeptide 2 (2.5 mg / kg / d); D is the positive drug intervention group: model + high-fat feed + rosuvastatin tablets (2.5 mg / kg / d). After 7 days of adaptive feeding with common feed, the right carotid artery intima was frostbited with liquid nitrogen in the model control group, drug intervention group and positive drug intervention group, and the model control group, drug intervention group and positive drug intervention group were fed with high-fat diet after operation , and at the same time administered intragastrically, and performed drug intervention treatment, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com