A method for the preparation of diazoalkanes
A technology for diazoalkane and alkyl, which is applied in the field of preparing the N-alkyl-N-nitroso compound, can solve problems such as environmental and waste disposal problems, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] Preparation of N-Alkyl-N-nitroso Compounds
[0059]
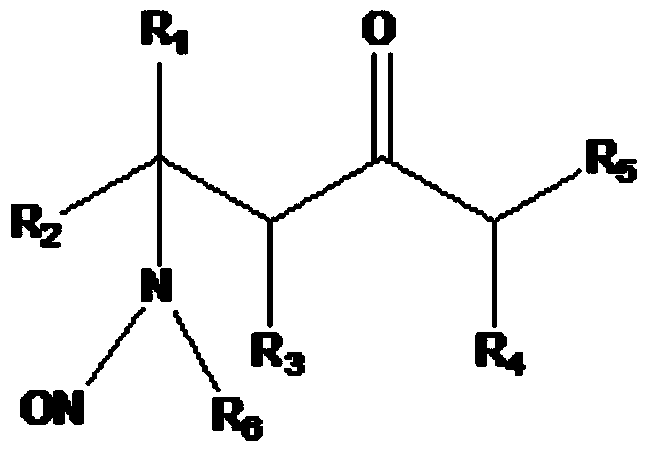
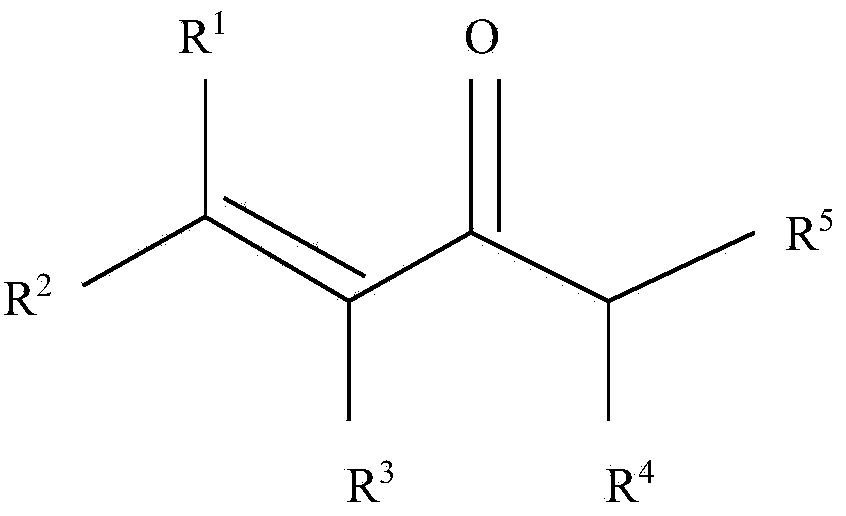
[0060] The general method of adding an aliphatic amine to a starting material to form an intermediate amine followed by addition of an acid and an alkali metal nitrite to make an N-alkyl-N-nitroso compound is well established in the art. Known. An exemplary method for preparing N-alkyl-N-nitroso compounds according to the present invention is outlined in Scheme 2 above. In the above embodiment, the N-alkyl-N-nitroso compound is N-nitroso-β-methylaminoisobutyl methyl ketone, and the starting material is mesityl oxide. The above-mentioned method can be carried out continuously or in batches.
[0061] One aspect of the invention involves the acidification of amines with tribasic acids. Any tribasic acid can be used, such as phosphoric or citric acid. In another embodiment, the tribasic acid is phosphoric acid. Preferably, the phosphoric acid is between 60% and 80% phosphoric acid in water, most preferably 75% ph...
Embodiment
[0080] Preparation of N-nitroso-β-methylaminoisobutyl methyl ketone
[0081] A 2 L glass reactor was equipped with a 500 mL addition funnel, stirrer, thermometer and cooling bath.
[0082] 1. Charge the reactor with 39.7% aqueous methylamine (230 g) and cool to 10°C.
[0083] 2. When the temperature reached 7°C, the addition of mesityl oxide (300 g) was started. The temperature is controlled between 10°C and 15°C. The total addition time was 57 minutes.
[0084] 3. Warm the clear light orange solution to 22°C over 5 minutes and then stir at this temperature for 60 minutes.
[0085] 4. Cool the solution to 10°C over 5 minutes. 75% phosphoric acid (310.2 g) was added over 60 minutes. Keep the temperature between 15°C and 20°C. During the addition, the mixture became more viscous. The pH at the end of the addition was 6.65.
[0086] 5. Add 30% aqueous sodium nitrite solution (771.3 g) between 10°C and 15°C over 8 minutes.
[0087] 6. Stir the mixture between 20°C and 25°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com