Virginiamycin microemulsion and preparation method thereof
A virginiamycin and microemulsion technology, applied in the field of medicine, can solve problems such as restricting popularization and application, and achieve the effects of strong operability, full absorption and utilization, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
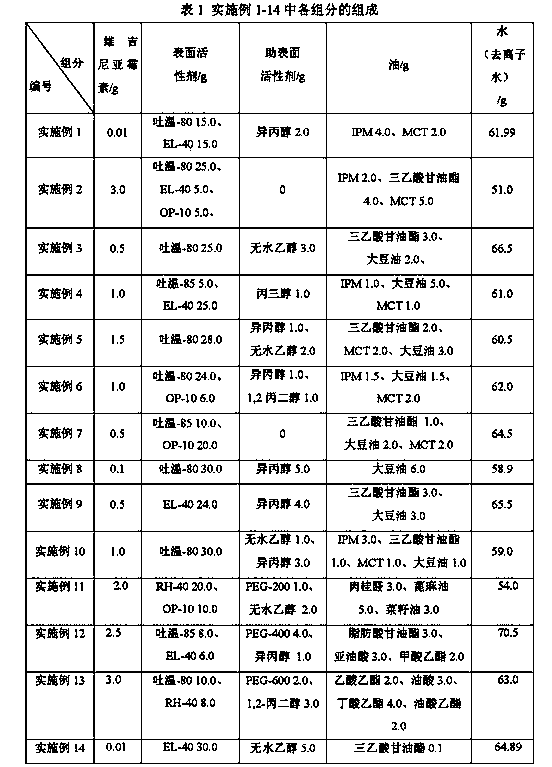
Embodiment 1-14
[0029] The preparation method of embodiment 1-14 virginiamycin microemulsion, comprises the following steps:
[0030] a) mixing virginiamycin with oil;
[0031] b) Add a co-surfactant to it, heat while stirring until the drug is completely dissolved, the heating temperature is ≤ 80°C, and the solution is clear and transparent by sampling;
[0032] c) Cool to room temperature, then add surfactant, stir evenly, the system remains clear and transparent, relatively viscous;
[0033] d) Slowly add deionized water, stir and mix while adding. At this time, the system is clear and transparent, and the sampling solution flows like a water sample.
Embodiment 15
[0035] The microemulsion products of Examples 1-14 were observed under a transmission electron microscope, and the scanning conditions were: HV=80.0kv; Direct Mag: 70 000×, figure 1 It is the transmission electron micrograph of the virginiamycin microemulsion that embodiment 1 makes, from figure 1 It can be seen that the virginiamycin droplets are spherical, with good dispersion and no adhesion, and the droplet diameters are distributed between 1 and 100 nm.
Embodiment 16
[0036] Example 16 Stability test
[0037] Get the microemulsion product of embodiment 1-14 to carry out time-lapse test, accelerated test, anti-freezing stability test and centrifuge stability test respectively, observe the stability of microemulsion of the present invention, confirm whether layering, turbidity or crystal are separated out etc. Instability occurs.
[0038] 1. Time-lapse test
[0039] The microemulsions of Examples 1-14 were stored for 6 months under the condition of natural changes at room temperature, and the properties and content changes of the microemulsions were investigated. The results showed that the appearance was persistently clear and transparent, and no demulsification such as turbidity, layering, and drug precipitation was found. , indicating good stability over time.
[0040] 2. Accelerated test
[0041] The microemulsions of Examples 1-14 were subpackaged in several glass bottles, placed in a temperature of 40°C after sealing, and stored ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com