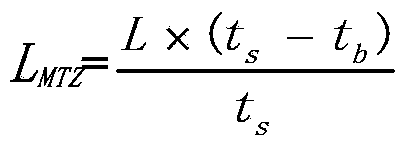Absorbent for chloride removal
An adsorbent, chloride technology, applied in other chemical processes, chemical instruments and methods, dispersed particle separation, etc., can solve problems such as difficult removal, low adsorption capacity of organic chlorides, leakage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
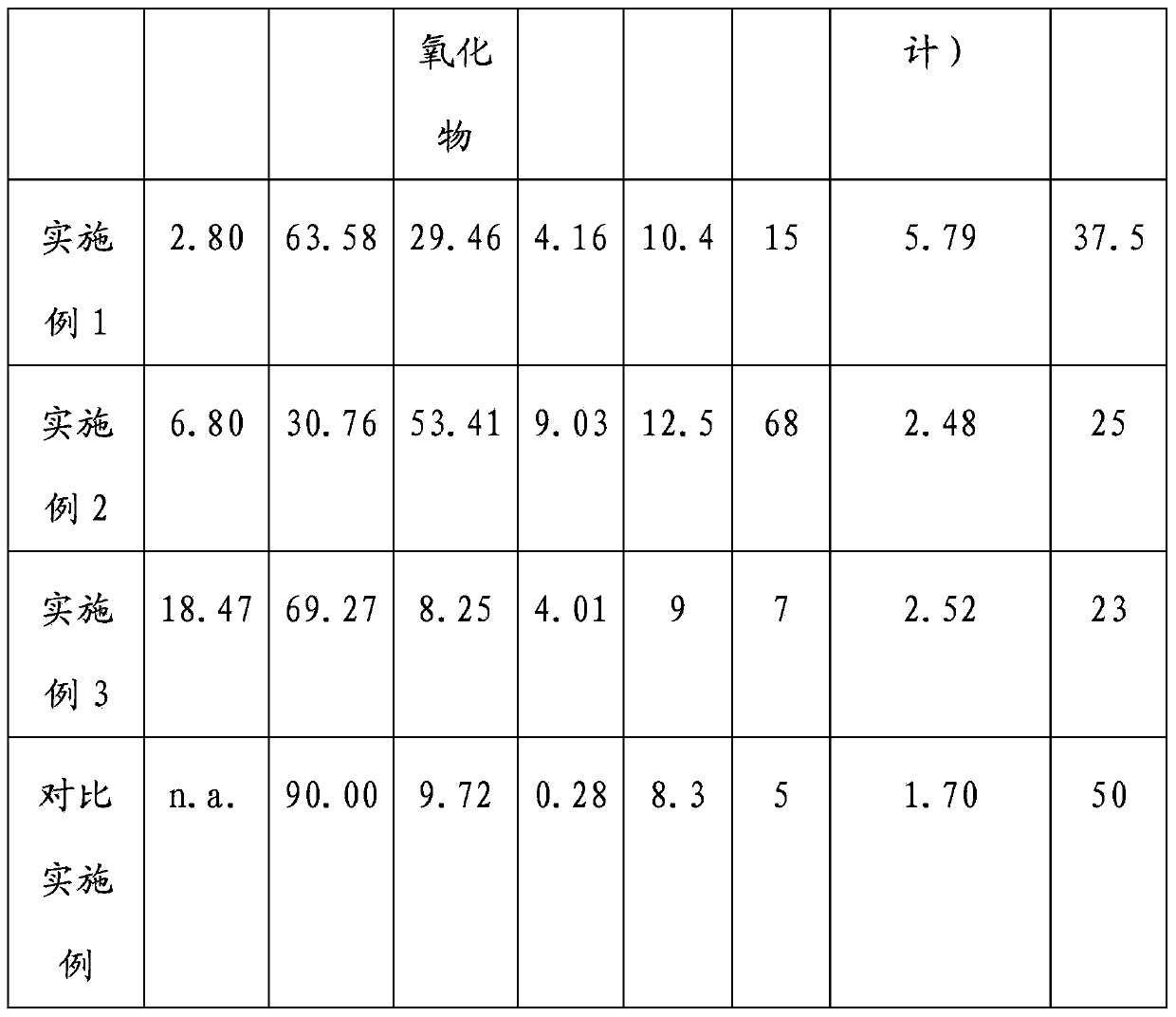
[0035] Sorbent preparation : 80 ml of an aqueous solution comprising 40% by weight of sodium aluminate and 7% by weight of calcium oxide is contacted with 60 g of activated carbon powder to obtain a wet impregnated product. The activated carbon powder is derived from corncobs and has a BET surface area of 763.2 m2 / g and a surface area of 35 cm 3 / 100g micropore capacity. The wet impregnated product was dried in an oven at 120°C for 2 hours and then calcined at 280°C for 4 hours in an air atmosphere. Based on the total weight of the adsorbent, the calcined product had an activated carbon content of 2.8%, an alumina content of 63.58%, a calcium oxide content of 8.57%, a sodium oxide content of 20.89%, and the remaining other contents.
[0036] Test for Chloride Removal : 1 kg of the adsorbent prepared in this way is packed into an adsorption container with a length-to-diameter ratio of 20. Hydrogen by-product gas from the catalytic reformer of the aromatics plant was fe...
Embodiment 2
[0040] Sorbent preparation : Dry mix 43.4g of activated carbon powder, 46.2g of sodium aluminate powder, 8.0g of calcium oxide powder and 2.2g of aluminum powder for 10 minutes, during which water is gradually added to the above at a ratio of water to powder of 0.25 ingredients. The activated carbon powder is derived from corncobs and has a BET surface area of 763.2 m2 / g and a surface area of 35 cm 3 / 100g micropore capacity. The mixture thus obtained was extruded to form cylindrical pellets of 3.5 mm before being dried in an oven at 120° C. for 2 hours and calcined in an air atmosphere at 300° C. for 3 hours. Based on the total weight of the adsorbent, the calcined product had an activated carbon content of 6.8%, an alumina content of 30.76%, a calcium oxide content of 22.65%, a sodium oxide content of 30.76%, and other remaining contents.
[0041] Test for Chloride Removal : Test in the same manner as in Example 1. The test results are shown in Table 1.
Embodiment 3
[0043] Sorbent preparation : Dry mix 79.4g of activated carbon powder, 12.6g of sodium aluminate powder, 5.8g of calcium oxide powder and 2.2g of aluminum powder for 10 minutes, during which water is gradually added to the above at a ratio of water to powder of 0.25 ingredients. The activated carbon powder is derived from corncobs and has a BET surface area of 763.2 m2 / g and a surface area of 35 cm 3 / 100g micropore capacity. The homogeneous mixture thus obtained was extruded to form cylindrical pellets of 3.5 mm before being dried in an oven at 120° C. for 2 hours and calcined at 300° C. in an air atmosphere for 3 hours. Based on the total weight of the adsorbent, the calcined product had an activated carbon content of 18.47%, an alumina content of 69.27%, a calcium oxide content of 4.02%, a sodium oxide content of 4.23%, and other residual contents.
[0044] Test for Chloride Removal : Test in the same manner as in Example 1. The test results are shown in Table 1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com