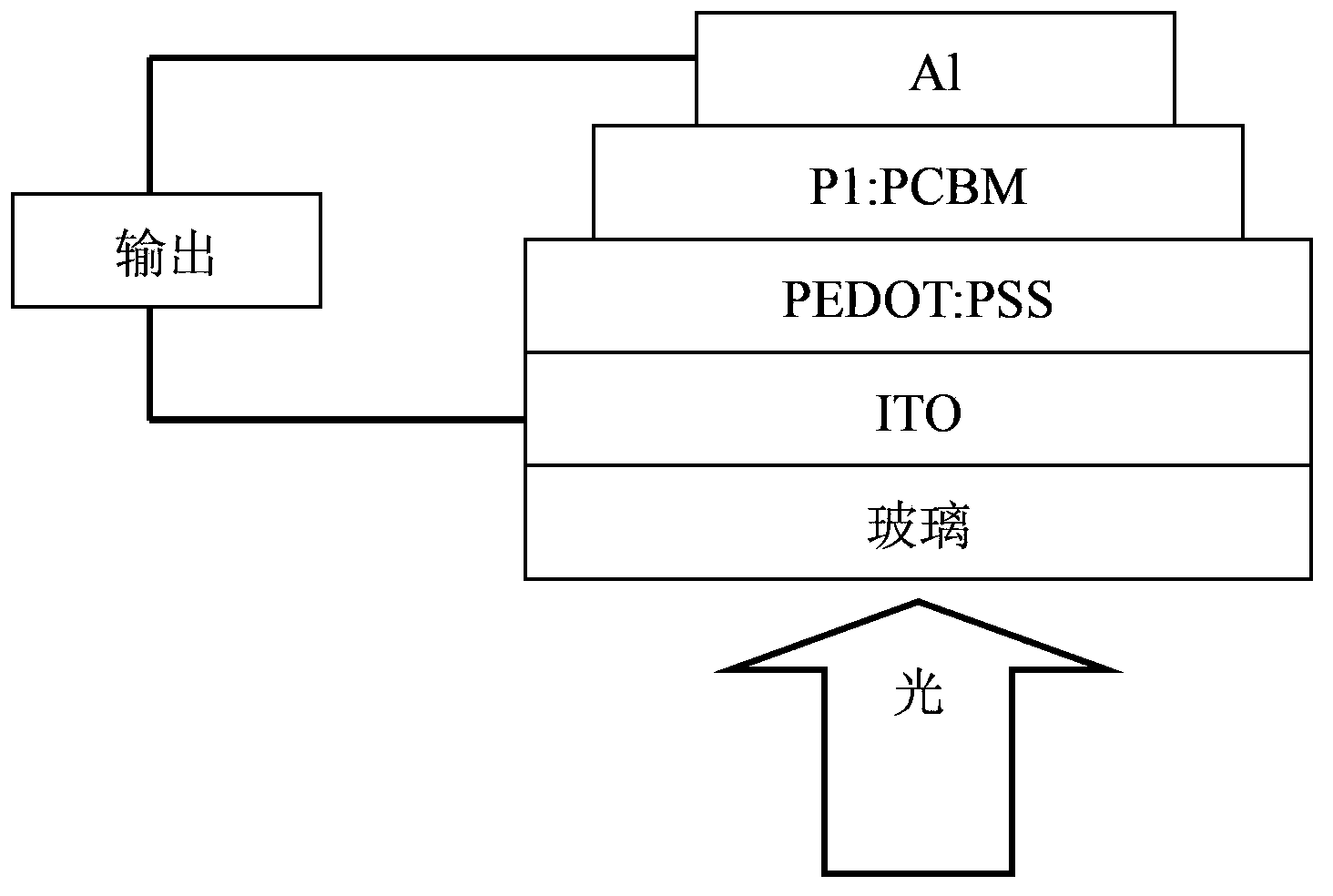
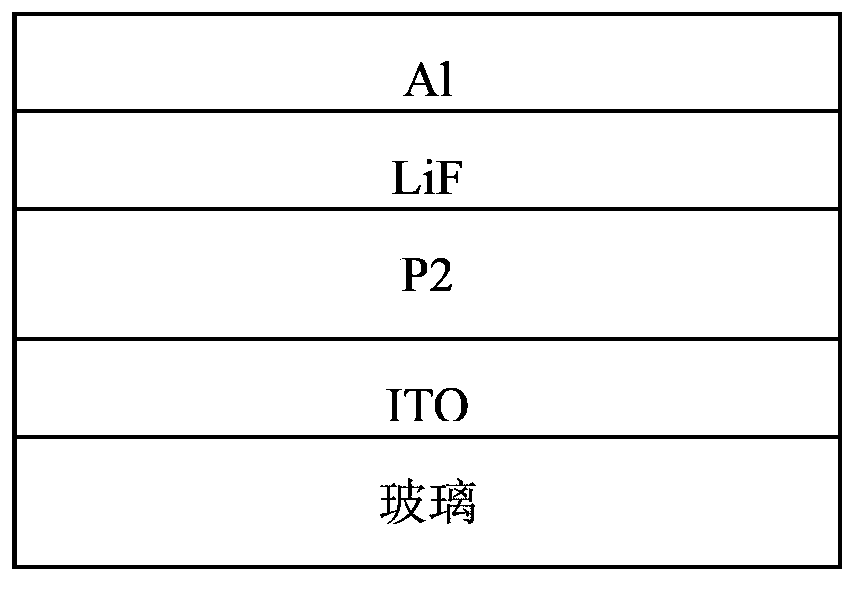
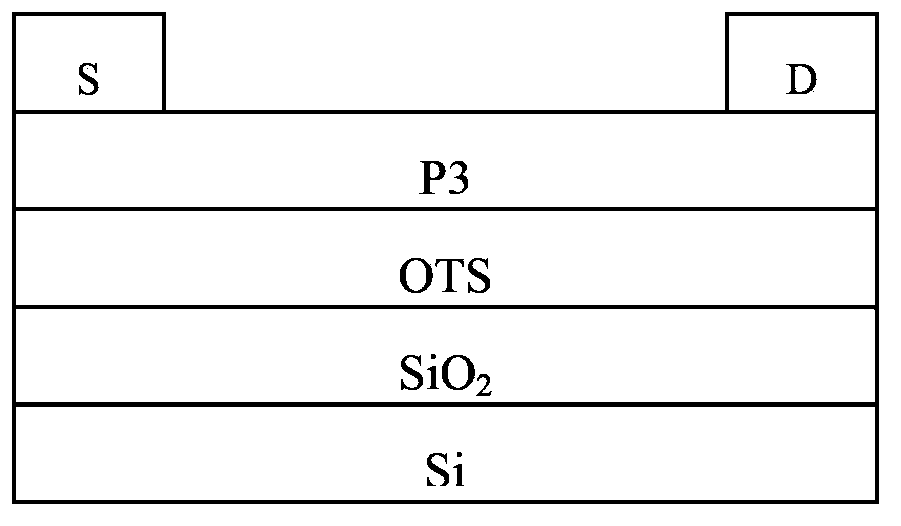
Conjugated polymer, preparation method and applications thereof
A technology of conjugated polymers and compounds, used in chemical instruments and methods, semiconductor/solid-state device manufacturing, organic chemistry, etc., can solve problems such as low photoelectric conversion efficiency, and achieve the effect of increasing the light absorption range and reducing the energy gap.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of above-mentioned conjugated polymer comprises the following steps:
[0045] a) provide the following compounds A and B;
[0046] Compound A is
[0047] Compound B is
[0048] b) Under an inert gas atmosphere, add the compound A and the compound B into an organic solvent at a molar ratio of 1:1.1-1.5, add a catalyst after dissolving, and then carry out a Stille coupling reaction at 60-120°C for 24- 72 hours, separated and purified to obtain the conjugated polymer (P);
[0049]
[0050] Among them, R 1 for C 1 ~C 16 the alkyl group, R 2 for C 1 ~C 16 the alkyl group, R 3 , R 4 H or C respectively 1 ~C 12 Alkyl group, n is a natural number between 5 and 60.
[0051] Described catalyst can be tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 ) or bis(triphenylphosphine)palladium dichloride (Pd(PPh 3 ) 2 Cl 2 );or
[0052] The catalyst can also be a mixture of organic palladium and organic phosphine ligands, the molar r...
Embodiment 1
[0070] The conjugated polymer disclosed in this example is specifically compound P1, and its structural formula is as follows:
[0071] n=5;
[0072] 1. Preparation of compound A1
[0073] 1) Synthesis of 2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene)
[0074]
[0075] Under nitrogen protection, 2.54g (60mmol) lithium chloride, 4.8g (10mmol) 2,5-dibromo-1,4-diiodobenzene (a), 287.5mg (0.25mmol) Pd were successively added to the reaction flask (PPh 3 ) 4 , 5g (50mmol) cuprous chloride, 3.59g (11mmol) 3-bromo-2-trimethyltinthiophene (b), 60ml tetrahydrofuran. After the reactant was stirred at room temperature for 1 h, the temperature was raised to 50° C. for 3 h. After cooling to room temperature, the solid produced by the reaction was filtered to obtain the crude product c, namely 2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene), with a yield of 40% , MS (EI) m / z: 558 (M + );
[0076] 2) Synthesis of compound d1
[0077]
[0078] Under argon protection, 5...
Embodiment 2
[0087] The conjugated polymer disclosed in this example is specifically compound P2, and its structural formula is as follows:
[0088] n=60;
[0089] One, the preparation of compound A2:
[0090] 1) Synthesis of 2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene)
[0091] Under nitrogen protection, 2.63g (62mmol) lithium chloride, 4.8g (10mmol) 2,5-dibromo-1,4-diiodobenzene (a), 0.7mg (0.001mmol) Pd were successively added to the reaction flask (PPh 3 ) 2 Cl 2 , 5.15g (52mmol) cuprous chloride, 4.89g (15mmol) 3-bromo-2-trimethyltinthiophene (b), 60ml DMF. The reactant was stirred at room temperature for 2 h, and then heated to 55° C. for 4 h. After cooling to room temperature, the solid produced by the reaction was filtered to obtain the crude product c, namely 2,2'-(2,5-dibromo-1,4-phenyl)bis(3-bromothiophene), with a yield of 39% , MS (EI) m / z: 558 (M + ); (see Example 1 for the reaction formula)
[0092] 2) Synthesis of compound d2
[0093]
[0094] Under nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com