Synthetic method of octadecyl erucyl amide
A technology of octadecyl erucamide and a synthesis method, which is applied in the field of synthesis of octadecyl erucamide, can solve the problems of poor activity stability, difficult product separation, long reaction time and the like, and saves solvents The effect of removal and product refining process, easy on-site operation and implementation, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
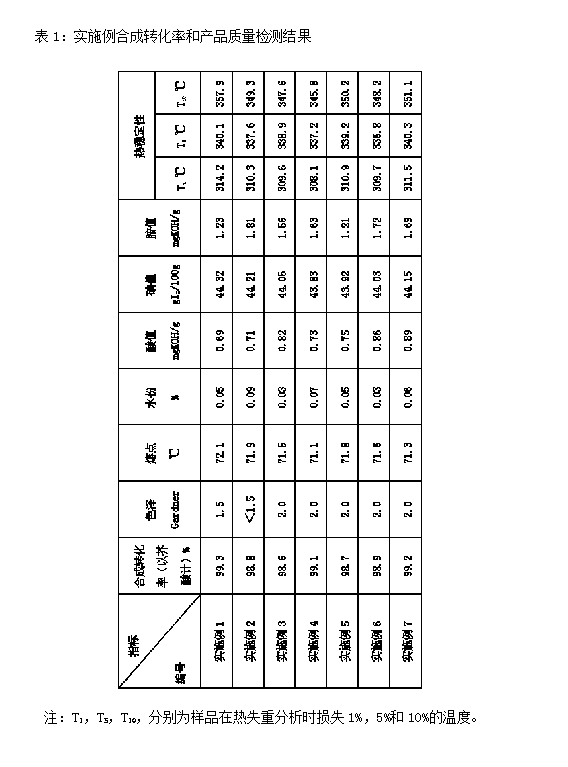
[0015] Add 560g of octadecylamine and 680g of erucic acid into the 3L reactor respectively, then raise the temperature to 130°C, start the stirring in the reactor, and remove the moisture in the reactor, then add 4.08g of catalyst, and continue to heat up to 190°C ℃ to start the reaction, control the reaction temperature at 190±1℃, the early stage (first 2h) reaction is evacuated under normal pressure under the protection of nitrogen; the later stage reaction is carried out under reduced pressure (vacuumization), and the reaction time is 3h and then cooled to 100℃ , and then filter the catalyst through a bag filter, then cool the filtrate, slice and shape it, and obtain a finished product. Then detect the synthetic conversion rate and product quality, the results are shown in Table 1.
[0016] Catalyst preparation: Dissolve 8g of phosphotungstic acid in 150ml of ethylene glycol and ethanol (1:1) mixed solvent to obtain a phosphotungstic acid solution; add 72g of chromatographi...
Embodiment 2
[0018] Add 600g of octadecylamine, 690g of erucic acid, and 4.83g of catalyst into a 3L reactor (composition of the catalyst: 15% silicotungstic acid, 15% metatitanic acid, 70% chromatography silica gel), method and implementation Example 1 is the same.
Embodiment 3
[0020] In 3L reactor, add 630g octadecylamine and 760g erucic acid, and 6.08g catalyst (catalyst component composition: phosphomolybdic acid 12%, metatitanic acid 25%, chromatography silica gel 63%), method and embodiment 1 same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com