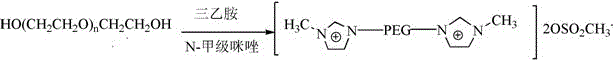A kind of PEG-functionalized bis-imidazolium cation temperature-controlled ionic liquid and its preparation method and application
A technology of bisimidazolium cation and ionic liquid is applied in the field of temperature-controlled ionic liquid of bisimidazolium cation and its preparation, which can solve the problems of increasing impurities in ionic liquid, polychlorinated by-products, polluting the environment, etc., and achieves simple preparation process, chlorination Less by-products and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
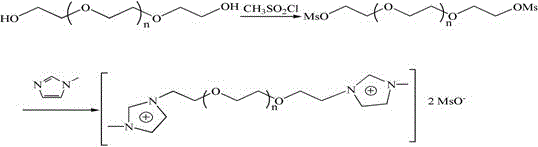
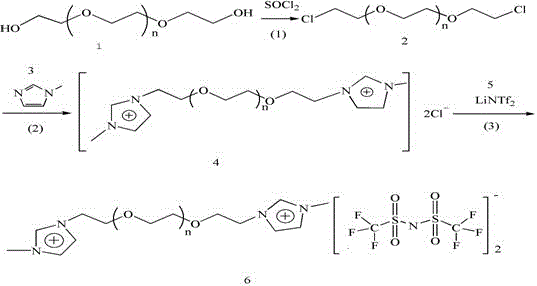
[0038] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous toluene to form solution 1, dissolve 62mmol of thionyl chloride and 62mmol of pyridine in 150mL of anhydrous toluene to form solution 2, and add solution 1 to solution 2 dropwise under nitrogen protection reaction at 60° C. for 2 hours, and then suction-filtered, an appropriate amount of anhydrous potassium carbonate was added to the filtrate to neutralize the acidic substances, suction-filtered, and rotary evaporated to obtain compound 2 (yield 90%).
[0039] Cl-PEG400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0040] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0041] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0042] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0043] (2) Under nitrogen protection, 20 mmol of compound 2 and 40 mmol of compound 3 were r...
Embodiment 2
[0054] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous chloroform to form solution 1, dissolve 93mmol of thionyl chloride and 93mmol of potassium carbonate in 150mL of anhydrous chloroform to form solution 2, and add solution 1 dropwise to the solution under nitrogen protection 2, reacted at 70°C for 3 hours, then suction filtered, added an appropriate amount of anhydrous potassium carbonate to the filtrate to neutralize acidic substances, suction filtered, and rotary evaporated to obtain compound 2 (yield 90%).
[0055] Cl-PEG 400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0056] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0057] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0058] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0059] (2) Under nitrogen protection, 20 mmol of compound 2 and 60 mmol of compound...
Embodiment 3
[0070] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous n-hexane to form solution 1, dissolve 124mmol of thionyl chloride and 124mmol of potassium bicarbonate in 150mL of anhydrous n-hexane to form solution 2, and dissolve 1 drop of the solution under nitrogen protection Added to solution 2, reacted at 80°C for 4 hours, then filtered with suction, added an appropriate amount of anhydrous potassium carbonate to the filtrate to neutralize acidic substances, filtered with suction, and rotary evaporated to obtain compound 2 (yield 90%).
[0071] Cl-PEG 400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0072] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0073] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0074] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0075] (2) Under nitrogen protection, 20 mmol of compound 2 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com