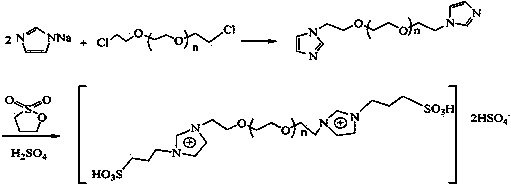
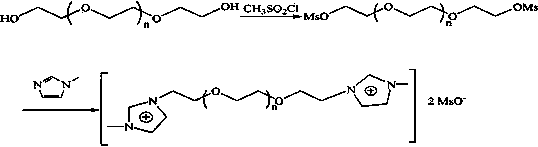
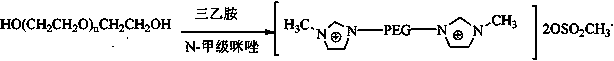PEG (polyethylene glycol) functionalized biimidazole cation temperature control ion liquid as well as preparation method and application thereof
A technology of bisimidazolium cation and ionic liquid is applied in the field of temperature-controlled ionic liquid of bisimidazolium cation and its preparation, which can solve the problems of increased impurities in ionic liquid, polychlorinated by-products, increased difficulty and the like, and achieves a simple preparation process, high chlorination Low by-products and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous toluene to form solution 1, dissolve 62mmol of thionyl chloride and 62mmol of pyridine in 150mL of anhydrous toluene to form solution 2, and add solution 1 to solution 2 dropwise under nitrogen protection , reacted at 60°C for 2 hours, and then filtered with suction. An appropriate amount of anhydrous potassium carbonate was added to the filtrate to neutralize the acidic substances, filtered with suction, and rotary evaporated to obtain compound 2 (yield: 90%).
[0039] Cl-PEG 400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0040] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0041] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0042] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0043] (2) Under nitrogen protection, 20 mmol of compound 2...
Embodiment 2
[0054] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous chloroform to form solution 1, dissolve 93mmol of thionyl chloride and 93mmol of potassium carbonate in 150mL of anhydrous chloroform to form solution 2, and add solution 1 dropwise to the solution under nitrogen protection 2, reacted at 70°C for 3 hours, then suction filtered, added an appropriate amount of anhydrous potassium carbonate to the filtrate to neutralize acidic substances, suction filtered, and rotary evaporated to obtain compound 2 (yield 90%).
[0055] Cl-PEG 400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0056] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0057] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0058] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0059] (2) Under nitrogen protection, 20 mmol of compound 2 and ...
Embodiment 3
[0070] (1) Dissolve 31mmol of compound 1 in 100mL of anhydrous n-hexane to form solution 1, dissolve 124mmol of thionyl chloride and 124mmol of potassium bicarbonate in 150mL of anhydrous n-hexane to form solution 2, and dissolve 1 drop of the solution under nitrogen protection Added to solution 2, reacted at 80°C for 4 hours, then suction filtered, added an appropriate amount of anhydrous potassium carbonate to the filtrate to neutralize acidic substances, suction filtered, and rotary evaporated to obtain compound 2 (yield 90%).
[0071] Cl-PEG 400 -Cl. 1 HNMR (D 2 O, δ / ppm ): 3.62-3.67(m35.1H(OCH 2 CH 2 ) 8.8 ) .
[0072] Cl-PEG 600 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.64-3.69(m49.2H,(OCH 2 CH 2 ) 12.3 ).
[0073] Cl-PEG 800 -Cl. 1 HNMR (D 2 O, δ / ppm ):3.65-3.76(m69.6H,(OCH 2 CH 2 ) 17.4 ).
[0074] Cl-PEG 1000 -Cl. 1 H-NMR ((D 2 O, δ / ppm): 3.66-3.79 (m88.4H, (OCH 2 CH 2 ) 22.1 ).
[0075] (2) Under nitrogen protection, 20 mmol of compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com