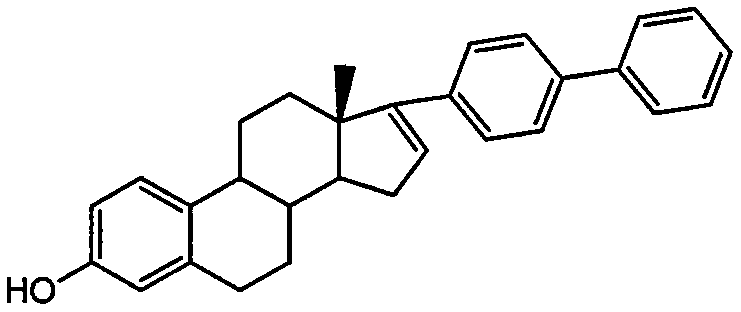
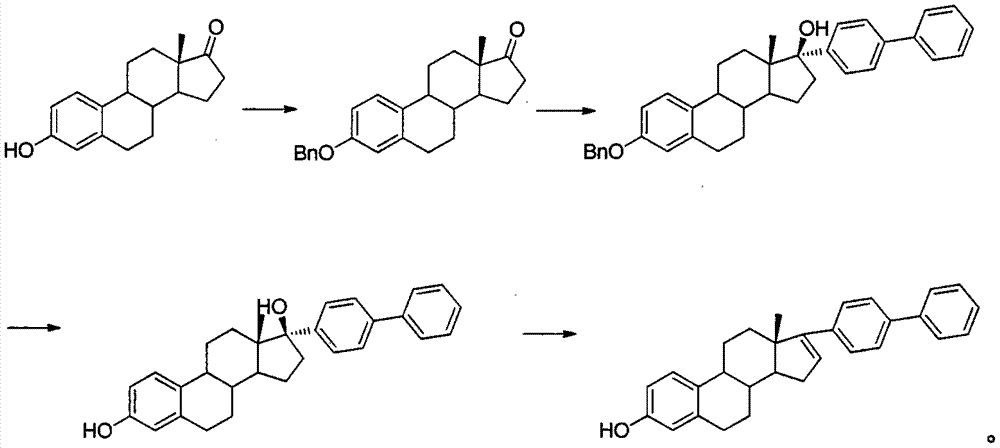
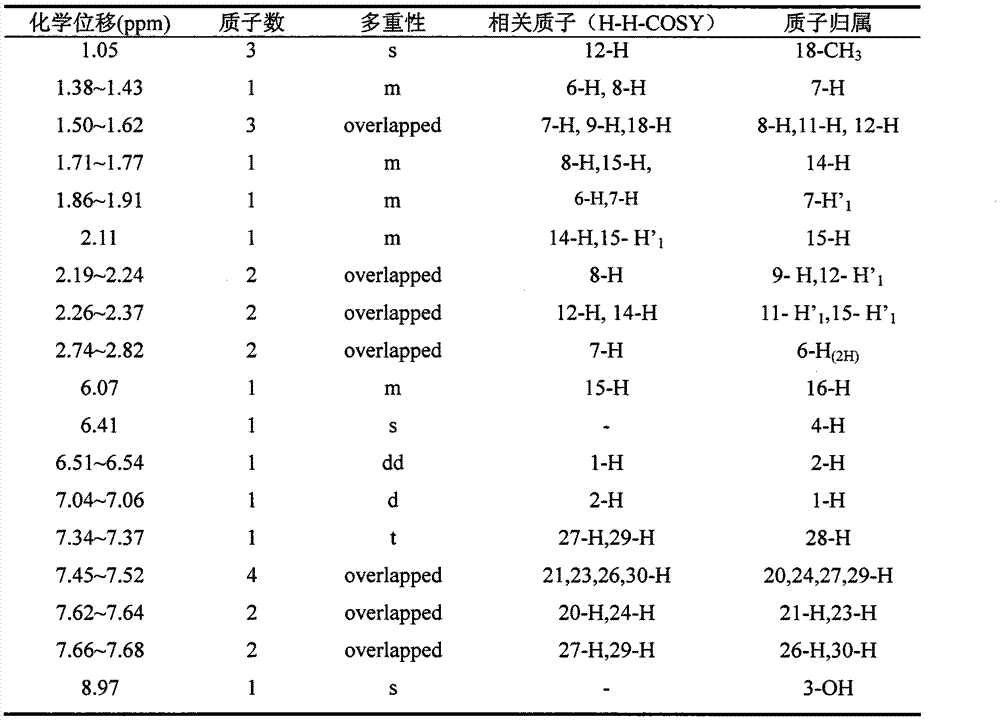
Estrone raw material medicine impurity, preparation method of estrone raw material medicine impurity, and application of estrone raw material medicine impurity being used as standard substance
A technology of estrone and benzylestrolone, which is applied in the field of medicinal chemistry, can solve the problems of qualitative analysis of estrone and fewer reports on impurity preparation processes, and achieve the effect of improving the quality of finished products and strengthening control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Into a 100 mL one-necked bottle equipped with a reflux condenser, add 2.7 g of estrone, 6.9 g of anhydrous potassium carbonate, 50 mL of DMF and 2.05 g of benzyl bromide. Stir at room temperature for 24h. The reaction solution was introduced into 500 mL of ice water, and filtered to obtain 2.1 g of Zener yellow solid 3-benzylestradiolone.
Embodiment 2
[0017] In a 250mL dry three-necked bottle equipped with a thermometer and a condenser, add 683mg of magnesium chips, a small amount of iodine and 20mL of anhydrous tetrahydrofuran. Stir at room temperature under nitrogen protection, and add 0.01 mL of dibromomethane into the reaction flask. Heating to reflux under vigorous stirring, the reddish-brown color of iodine receded and the reaction solution turned gray, indicating that the reaction was initiated, and 8.0 g of 4-diphenyl bromide was continued to be added dropwise. Continue to reflux for 45 min after the dropwise addition.
[0018] The reaction solution was cooled to room temperature, and a solution of 3-benzylestradiolone (5.41 g) in tetrahydrofuran (30 mL) was added dropwise, and stirring was continued for 6 h after the addition was completed. A sufficient amount of saturated ammonium chloride solution was added dropwise to destroy the reaction, filtered, and the organic phase was separated and concentrated to drynes...
Embodiment 3
[0020] Put 4.5g of 3-benzyl-17-(4'-phenylphenyl)-estra-1,3,5(10)-triene-3,17β-diol into the reaction bottle, 30mL tetrahydrofuran, 0.2g palladium Carbon was replaced with hydrogen and stirred overnight at room temperature. Filtrate, evaporate the filtrate to dryness, and purify by column chromatography to obtain 3 g of light yellow solid 17-(4'-phenylphenyl)-estra-1,3,5(10)-triene-3,17-di-ol .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com