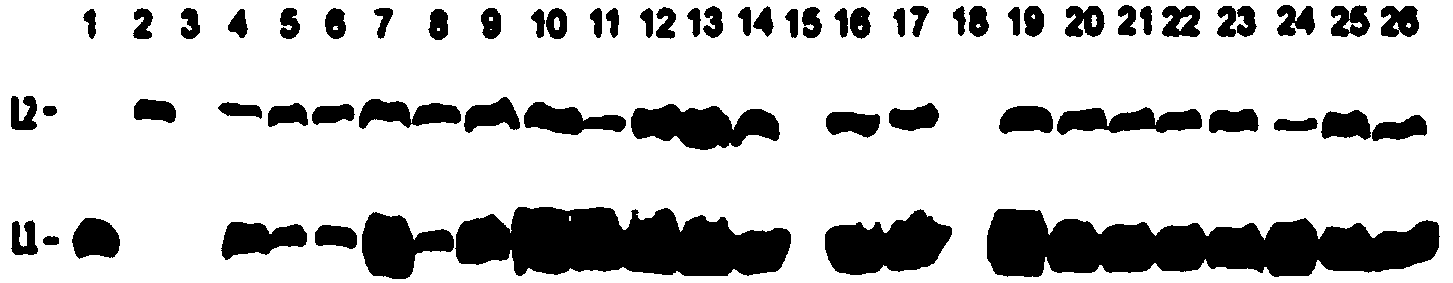Human papilloma virus L1L2 capsid protein interaction site and applications thereof
A technology of L1 protein and site, applied in the field of molecular virology, can solve problems such as limited application value and lack of experimental data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Computer analysis predicts the interaction site of HPV16L1 and HPV16L2
[0059] The Discovery Studio software used for protein structure analysis and simulation in this laboratory was purchased from Accelrys, USA. Discovery Studio software was used to analyze the surface amino acids of HPVL1 pentamer that might interact with L2, and the amino acid solvent accessibility surfaces (solvent accessibility surfaces, SAS) were evaluated. SAS represents the ratio of the surface area of the amino acid that can be exposed to the solvent to the surface area of the entire amino acid, which is an index that reflects the degree of exposure of the amino acid. The larger the value, the higher the degree of exposure of the amino acid to the surface of the protein structure (Le Grand, S.M., Eyrand, V .An algorithm for the representation and computatio of suermolecular surface and volumes. J.Comp.Chen.1989,10:868-871). Among them, 23 sites were screened out, and their...
Embodiment 2
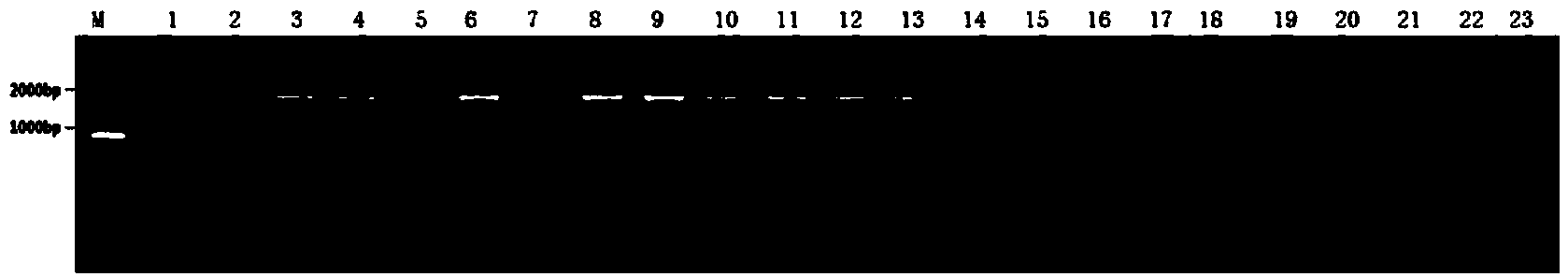
[0064] Embodiment 2: Construction of 23 pAAV-16L1 site mutation plasmids
[0065]Gene cloning is carried out by site-directed mutagenesis PCR reaction, using the pAAV-HPV16L1 plasmid constructed in this laboratory (gifted by Professor John T. Schiller of NIH, wherein the DNA sequence of HPV16L1 is as SEQ NO: 25 of the present invention) as a template (abbreviated as H16L1), the primers and products of each PCR reaction are shown in Table 2, use the primers designed in Table 3 to carry out PCR amplification, and perform PCR reaction in PCR instrument (Biometra, T3), and the amplification conditions are set as:
[0066] Denaturation at 94°C for 10 minutes, 25 cycles (denaturation at 94°C for 50 seconds, annealing at a specified temperature for a certain time, extension at 72°C for 6 minutes), and finally extension at 72°C for 10 minutes.
[0067] The amplified product was added with 2 μL DpnI restriction endonuclease, treated at 37° C. for 60 minutes, and then transformed into...
Embodiment 3
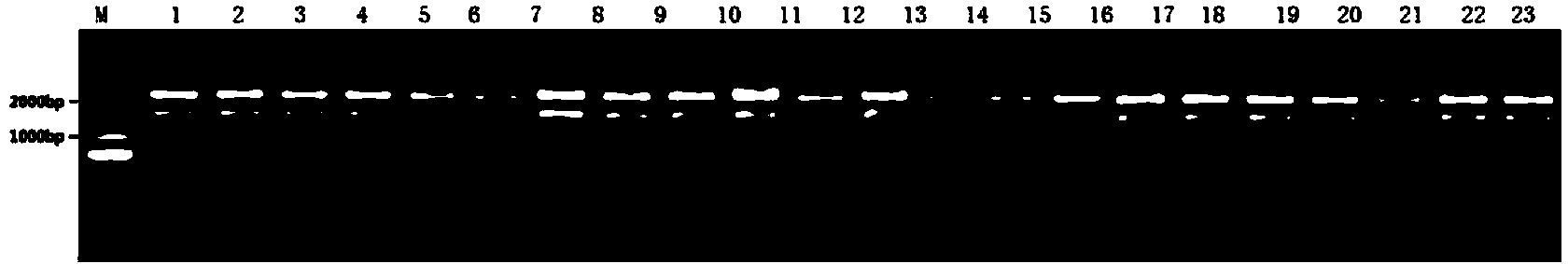
[0077] Embodiment 3: preparation of 23 HPV16 mutant pseudoviruses
[0078] The wild-type pAAV-16L1 plasmid obtained in Example 2 or 23 mutant pAAV-16L1 plasmids and pAAV-HPV16L2 plasmids (gifted by Professor John T. Schiller of NIH; those skilled in the art can construct by themselves, respectively) using calcium phosphate transfection method Other blank plasmids can also be used for enzyme digestion and recombination) and the plasmid pcDNA3.1-EGFP containing the reporter gene (for the construction method, see Lu Wuxun et al., Acta Biological Engineering, 2006, volume 22: 990-995) each with a total of 40 μg 293FT cells (purchased from Invitrogen) cultured in a 10 cm cell culture dish were transfected to prepare pseudoviruses or mutated pseudoviruses. Regarding the preparation of pseudoviruses, refer to Lu Wuxun et al., Acta Biological Engineering, 2006, volume 22: pages 990-995, and also refer to the following steps:
[0079] Add 40 μg each of the wild-type or mutant pAAV-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com