Gene chips and kits for detecting foot-and-mouth disease virus and swine fever virus
A technology for foot-and-mouth disease virus and swine fever virus, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, and microbial determination/inspection, etc. There are no problems such as chip specificity and sensitivity test, and the effect of good clinical application prospect, rapid detection and strong specificity is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 Detection method of the present invention
[0060] 1. Materials and instruments
[0061] The same experimental materials and instruments as mentioned above.
[0062] 2. Experimental method
[0063] 2.1 Design and synthesis of PCR primers and detection probes
[0064] Log in to GenBank to obtain the gene sequences of representative strains of FMDV and CSFV main genotypes (serotypes), use the MegAlign module of DNAStar software to conduct homology analysis, select conserved genes as detection target regions, and use Oligo7.0 software to design Amplification primers and oligonucleotide probes. When labeling samples, the downstream primers are primers modified by Cy3 at the 5' end; PolyT15 linking arms are added to the 5' end of the oligonucleotide probe and aminated. The primer sequences are shown in Table 1, and the oligonucleotide probes are shown in Table 2. After Blast comparison, they were sent to Shanghai Sangong for synthesis.
[0065] Table 1: Pri...
Embodiment 2
[0145] Embodiment 2 specificity test
[0146] 1. Test method
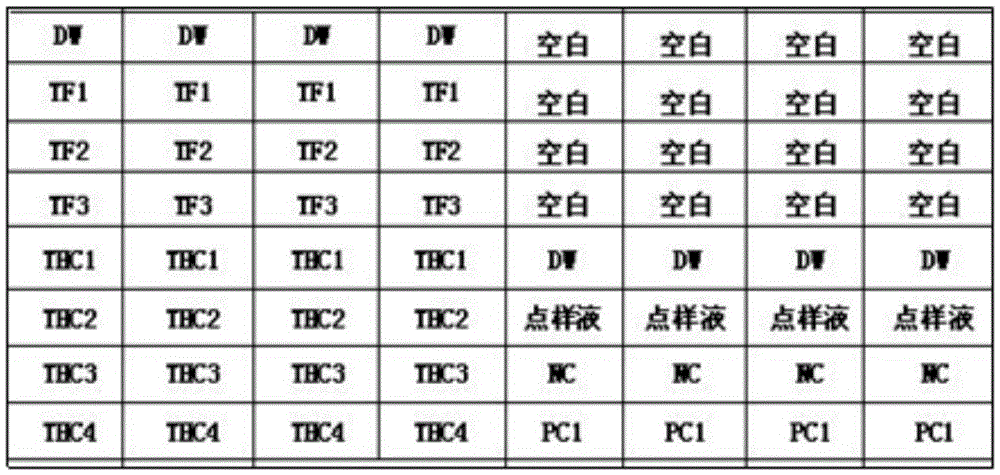
[0147] Detection of porcine circovirus (PCV-2), porcine parvovirus (PPV), pseudorabies virus (PRV), porcine Japanese encephalitis virus (JEV) and porcine epidemic diarrhea virus (PEDV) by multiplex asymmetric RT-PCR , porcine transmissible gastroenteritis virus (TGEV), bovine viral diarrhea / mucosal disease virus (BVDV) nucleic acid template amplification markers, and the amplified products were detected with the gene chip prepared in Example 1 respectively to evaluate its specificity.
[0148] The method of multiplex RT-PCR amplification is as follows:
[0149] Extract the nucleic acid of the sample to be tested (use the RNA column extraction kit and DNA column extraction kit of Beijing Century Yuanheng Company to extract the nucleic acid of the corresponding virus), multiplex RT-PCR amplification, and the reaction system is: use OneStepPrimeScriptTMRT-PCRKit amplification reagent Box, Buffer III (2x) 12.5 μL, En...
Embodiment 3
[0156] Embodiment 3 sensitivity test
[0157] 1. Test method
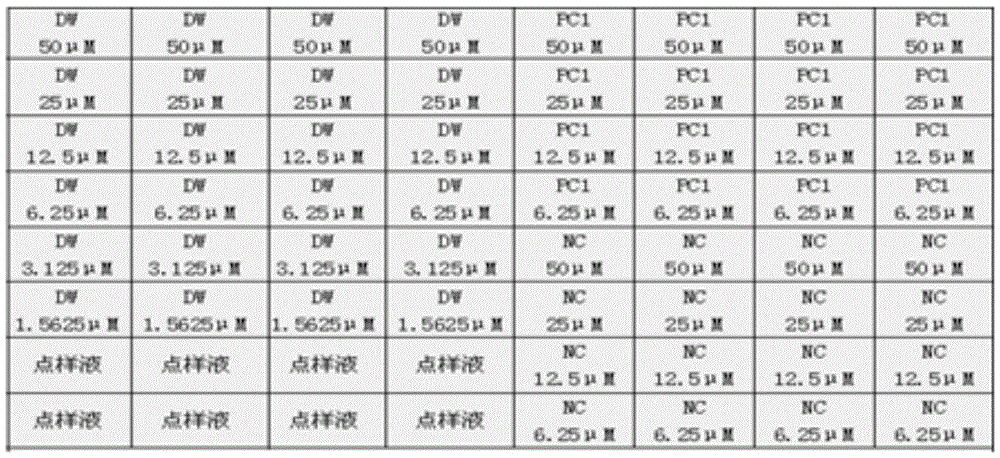
[0158] According to the method of Example 1, the recombinant plasmid PMD3D (8.34 × 10 9 copies / μL), PMD-W (1.06×10 9 copies / μL), PMD-C (9.02×10 9 copies / μL) were diluted 10×, amplified by multiple asymmetric RT-PCR, and the amplified products were detected by gene chip to evaluate the sensitivity of the detection gene chip system.
[0159] The multiplex RT-PCR amplification method and gene chip detection method are the same as in Example 2.
[0160] 2. Results
[0161] Adopt the method of the present invention to detect plasmid PMD3D, the minimum detection concentration can reach 8.34 * 10 2 copies / μL, test results see Figure 16 .
[0162] Adopt the method of the present invention to detect plasmid PMD-W, the minimum detection concentration can reach 1.06 * 10 2 copies / μL, test results see Figure 17 .
[0163] Adopt the method of the present invention to detect plasmid PMD-C, the minimum detection conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com