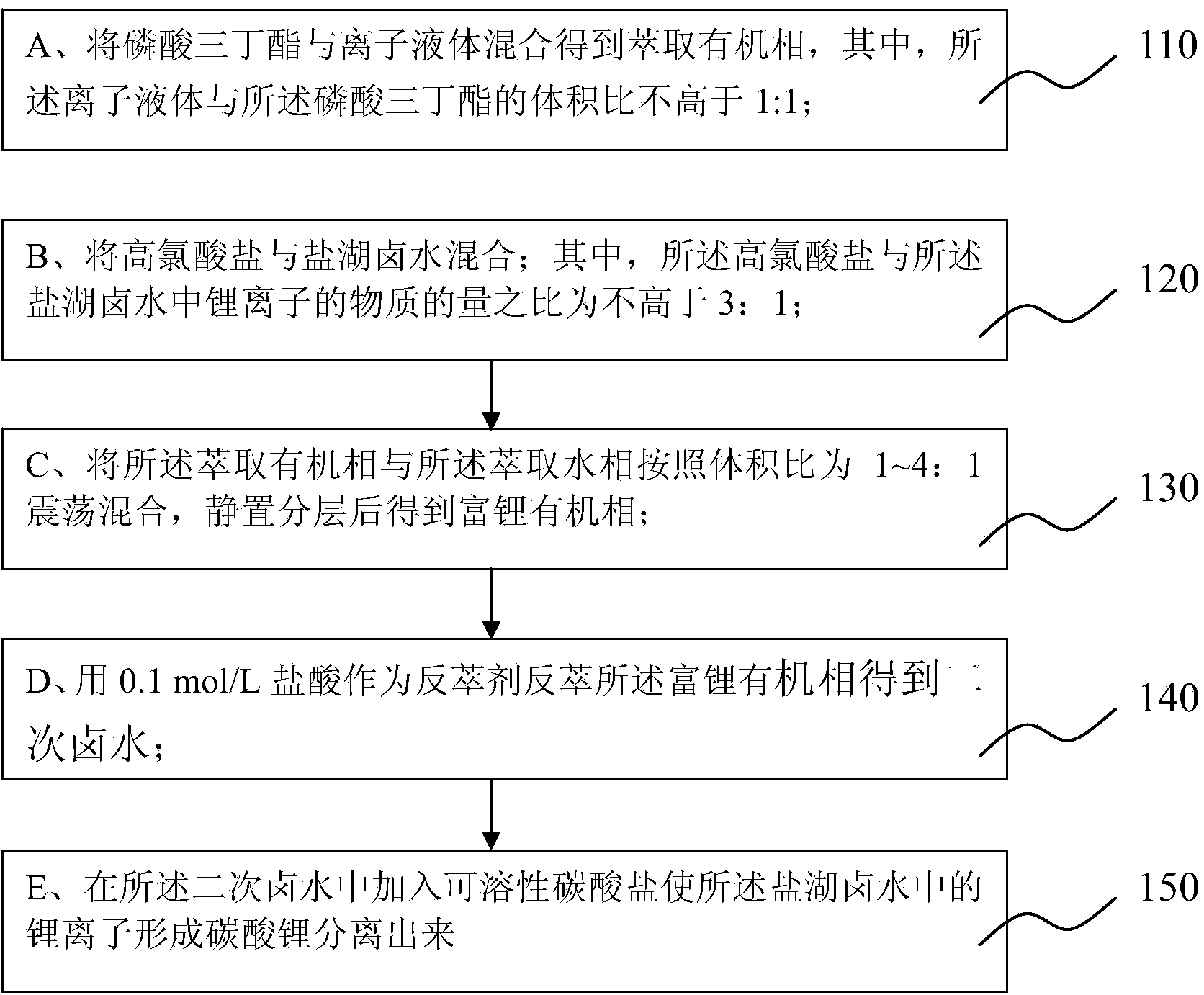
Method for separating lithium from salt lake brine
A salt lake brine and lithium-rich technology, applied in the field of extraction chemistry and chemical industry, can solve the problems of environmental pollution, difficulty in separating the aqueous phase and the organic phase in the extraction system, etc., and achieve the effects of avoiding environmental pollution, good practical value, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Adopt the method for extracting lithium from salt lake brine of the present invention, at first, add NaClO in the salt lake brine shown in Table 1 4 Solid, so that n(ClO4-):n(Li+)=2:1, mix evenly to configure the extraction water phase, filter to remove NaCl produced by the salting-out effect, and filter the water phase for later use. Among them, since the extraction system of this embodiment does not use ferric chloride, there is no need to add strong acid to adjust the pH value of the brine to prevent the hydrolysis of ferric ions and avoid the problem of three phases.
[0036] Then the volume ratio of the preparation is 80%TBP-20%[C 4 mim][PF 6 ] for the extraction of the organic phase.
[0037] The extracted organic phase and the extracted aqueous phase were added to the separatory funnel according to the preset volume ratio (O / A) in Table 2, shaken at 20°C for 10 minutes, and stood for 20 minutes to separate the two phases to obtain the lithium-rich organic phase...
Embodiment 2
[0044] In the description of this embodiment, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between this embodiment and Example 1 is: n(ClO4-):n(Li+)=1:1, O / A=1:1, and then the volume ratio is 90%TBP-10%[C 4 mim][PF 6 ] to extract the organic phase, and adjust the different shaking times to obtain the extraction efficiency as shown in Table 3.
[0045] Table 3 Comparison of extraction efficiencies at different shaking times
[0046] Shock time (min)
[0047] Table 3 illustrates that the present embodiment has been substantially close to the extraction equilibrium within the shaking time of 8 minutes. Therefore, in combination with faster extraction balance and higher extraction efficiency, 8-10 minutes is selected as the better shaking time.
Embodiment 3
[0049] The organic phase obtained under the ratio O / A=2 in Example 1 was subjected to a stripping experiment: the volume ratio (A / O) of the hydrochloric acid to the lithium-rich organic phase was controlled as shown in Table 4, and different stripping ratios were adjusted. (A / O) obtained different stripping efficiencies are shown in Table 3.
[0050] Table 4 Different extraction efficiencies of different back extractions (at a temperature of 20°C)
[0051] A / O
[0052] Table 4 illustrates that stripping efficiency increases with the increase of stripping ratio, and the increase of aqueous phase volume is beneficial to Li + reversed phase extraction. Further, the stripping ratio (A / O) is preferably in the range of 2 to 3, and the stripping efficiency can reach more than 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com