Ratiometric fluorescent probe for detection of hydrogen sulfide and preparation method of ratio-dependent fluorescent probe
A fluorescent probe and ratiometric technology, applied in the field of analysis and detection, can solve problems such as being susceptible to interference and difficult to guarantee detection accuracy, and achieve the effect of easy aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
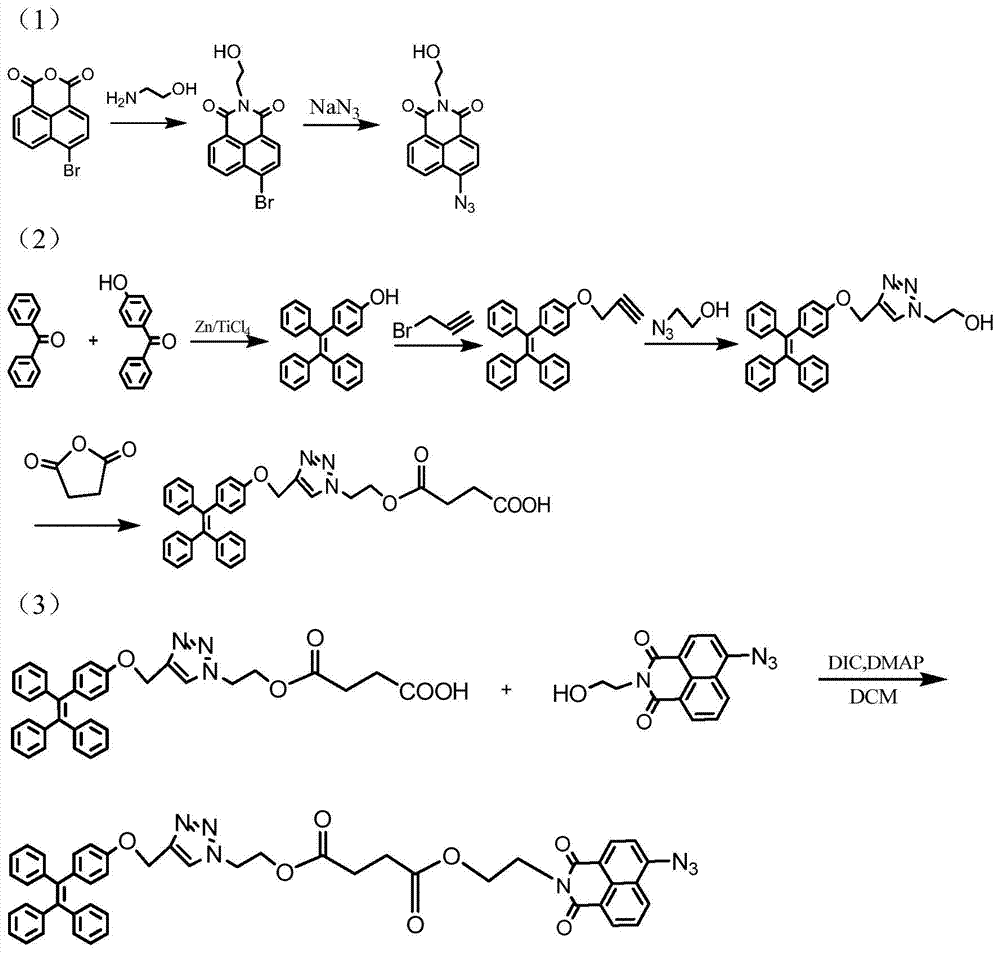
[0041] Embodiment 1: Preparation process 1 of probe compound TPE-NAP-N3
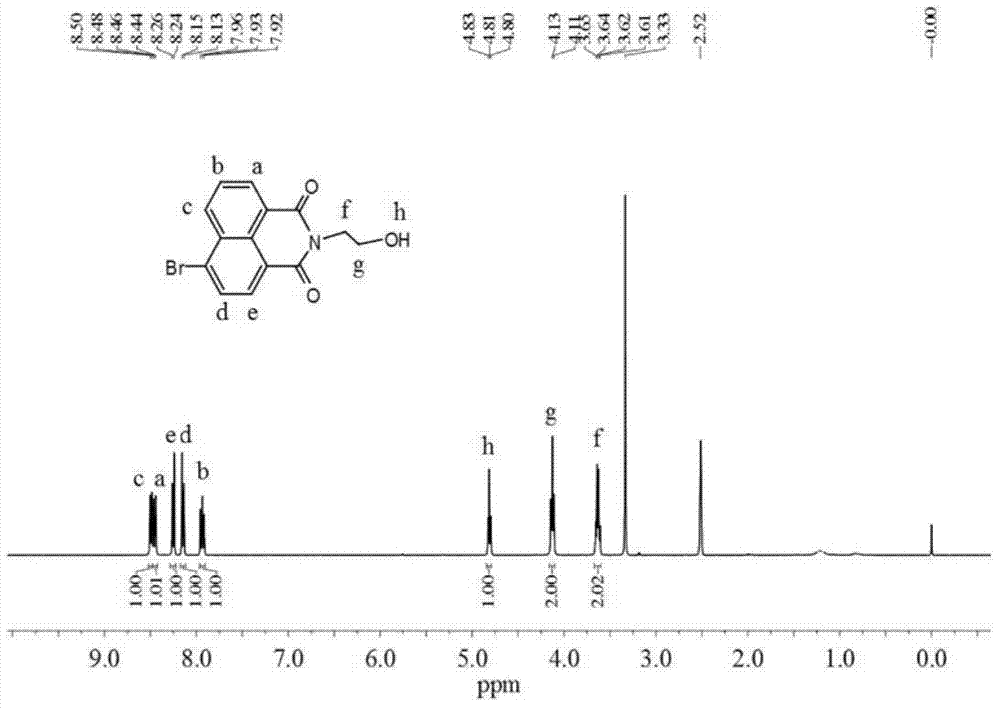
[0042] 1) 1305mg of 4-bromo-1,8-naphthalic anhydride (4.71mmol) was dissolved in 108mL of ethanol, and 317mg of 2-aminoethanol (5.19mmol) was slowly added dropwise under nitrogen protection. Under stirring, the The mixed solution was heated to reflux; after cooling to room temperature, the precipitate was filtered and collected, and the precipitate was recrystallized with ethanol to obtain 1254 mg of white solid 6-bromo-2-(2-hydroxyethyl)-benzisoquinoline-dione (83.2% yield). By H NMR spectroscopy ( figure 2 ) to characterize the product, 1 H NMR (400MHz, DMSO-d 6 ,δppm):8.48-8.50(d,1H),8.44-8.46(d,1H),8.24-8.26(d,1H),8.13-8.15(d,1H),7.92-7.96(t,1H),4.80 -4.83(t,1H), 4.11-4.13(t,2H), 3.62-3.65(t,2H). Among them, 8.48ppm, 8.44ppm, 8.24ppm, 8.13ppm and 7.92ppm correspond to the characteristic peaks of protons on the naphthalene ring, 4.81ppm corresponds to the characteristic peaks of hydroxyl protons...
Embodiment 2
[0049] Embodiment 2: Preparation process 2 of probe compound TPE-NAP-N3
[0050] 1) 2610mg of 4-bromo-1,8-naphthalic anhydride (9.42mmol) was dissolved in 188mL of ethanol, and 692mg of 2-aminoethanol (11.34mmol) was slowly added dropwise under nitrogen protection. Under stirring, the The mixed solution was heated to reflux; after cooling to room temperature, the precipitate was filtered and collected, and the precipitate was recrystallized with ethanol to obtain 2500 mg of white solid 6-bromo-2-(2-hydroxyethyl)-benzisoquinoline-dione (82.9% yield).
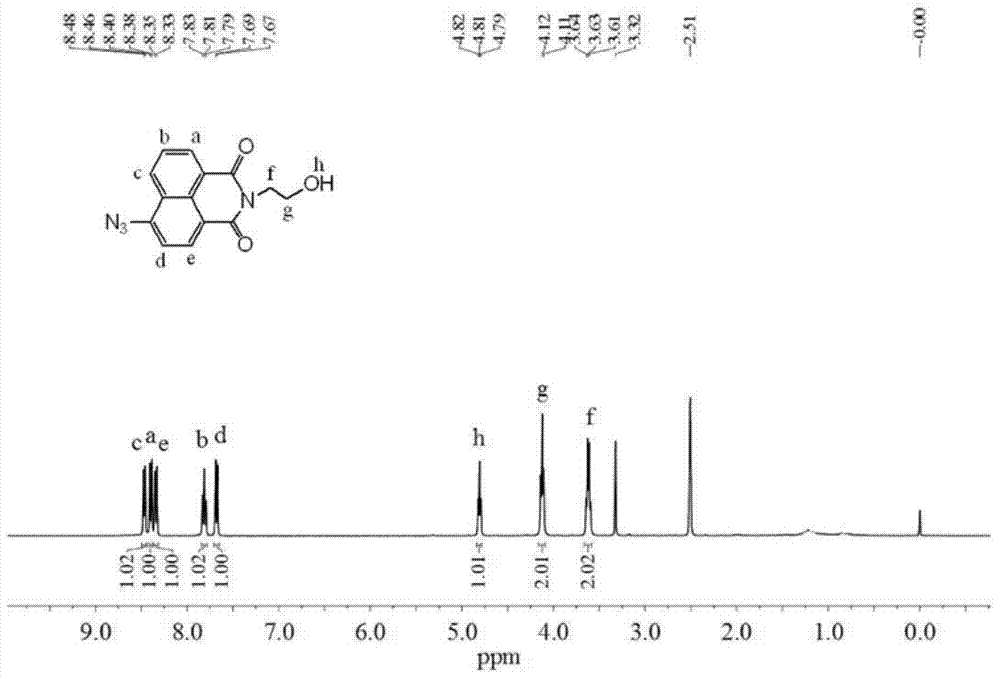
[0051] Dissolve 480mg of the above white solid (1.5mmol) and 975mg of sodium azide (15mmol) in 18mL of N,N-dimethylformamide, heat the solution to 110°C with stirring and keep it for 9 hours; cool to room temperature , adding 60 mL of deionized water, and then extracting with 300 mL of ethyl acetate. Collect the organic phase, wash the organic phase with brine, dry with 150 mg of anhydrous magnesium sulfate, and filter; the org...
Embodiment 3
[0058] Embodiment 3: Preparation process 3 of probe compound TPE-NAP-N3
[0059] 1) 870mg of 4-bromo-1,8-naphthalic anhydride (3.14mmol) was dissolved in 67mL of ethanol, and 220mg of 2-aminoethanol (3.61mmol) was slowly added dropwise under nitrogen protection. Under stirring, the The mixed solution was heated to reflux; after cooling to room temperature, the precipitate was collected by filtration, and the precipitate was recrystallized from ethanol to obtain 839 mg of white solid 6-bromo-2-(2-hydroxyethyl)-benzisoquinoline-dione (83.5% yield).
[0060] Dissolve 160mg of the above white solid (0.5mmol) and 325mg of sodium azide (5.5mmol) in 5.5mL of N,N-dimethylformamide, and heat the solution to 100°C under stirring conditions and keep it for 8 hours; cool After reaching room temperature, 30 mL of deionized water was added, followed by extraction with 200 mL of ethyl acetate. Collect the organic phase, wash the organic phase with brine, dry with 100 mg of anhydrous magnes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com