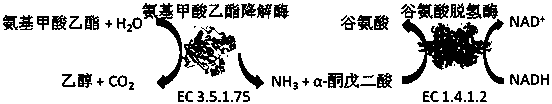
Spectrophotometric method for quickly detecting content of ethyl carbamate
A kind of urethane, content technology, applied in the field of food safety detection, analytical chemistry, can solve the problem of no reported enzyme sensor and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
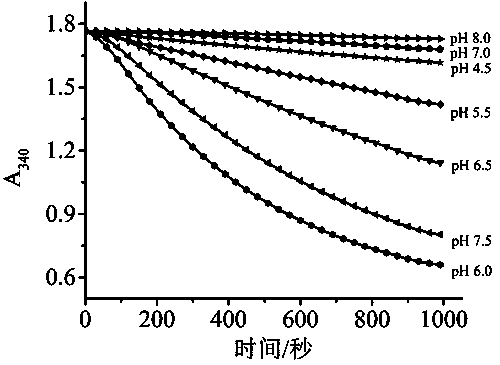
[0025] Example 1 Determination of the optimal reaction buffer system for the dual enzyme coupling system
[0026] Before using the dual-enzyme coupling system to detect the content of ethyl carbamate, the optimal reaction buffer system of the dual-enzyme system of ethyl carbamate degrading enzyme and glutamate dehydrogenase was optimized first. The urethane degrading enzyme used in the present invention can maximize its catalytic activity in an acidic environment, while the glutamic acid dehydrogenase has a higher catalytic activity in a moderately alkaline condition. The two catalyze two-step cascade reactions in the same reaction system. The type of buffer and pH are different, and the microenvironment of the enzyme active center and the catalytic reaction rate will change.
[0027] First prepare a concentration of 25mmol L -1 citrate buffer (pH 4.5, 5.5), phosphate buffer (pH 6.0, 6.5, 7.0) and Tris-HCl buffer (pH 7.5, 8.0), and use the corresponding pH buffer to dissolve ...
Embodiment 2
[0028] Example 2 Determination of Optimum Addition of Urethane Degrading Enzyme and Glutamate Dehydrogenase in Dual Enzyme Coupling System
[0029] In the optimal reaction buffer system, when the amount of glutamate dehydrogenase and other reaction substrates was kept constant, the enzyme activities added to the system were 4, 8, 12 and 16 U·mL -1 The urethane degrading enzyme was used, and then the mixed solution was placed at 340 nm to detect the change of absorbance value with time. With the increase of the enzymatic activity of ethyl carbamate degrading enzyme in the reaction system, the rate of decomposing EC to produce ammonia is accelerated, and the rate of glutamate dehydrogenase using ammonia to convert α-ketoglutarate is also accelerated, so the oxidation rate of NADH also increases. Gradually accelerated, manifested as an increase in the change in absorbance at 340 nm. Enzyme activity reaches 16 U·mL -1 After that, the change of the absorbance value at 340 nm has ...
Embodiment 3
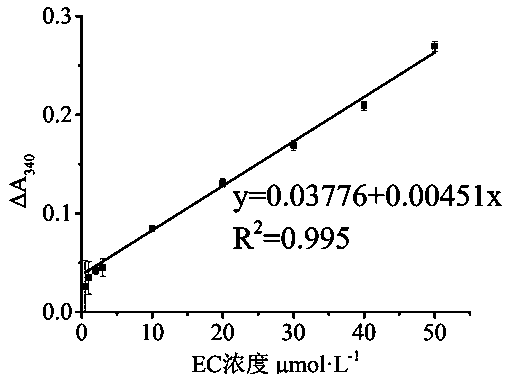
[0031] The establishment of embodiment 3 spectrophotometric method ethyl carbamate concentration standard curve
[0032] In the optimal reaction buffer system, in the double-enzyme coupling system with the determined optimal addition of urethane degrading enzyme and glutamate dehydrogenase, 540mmol·L -1 α-ketoglutarate, and 7.5mmol·L -1 In NADH solution, its A was measured 340 is 0.46733, and the concentrations of added urethane are 0, 0.01, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 30, 40, 50 μmol L -1 After the A 340 are 0.46733, 0.48167, 0.48567, 0.49133, 0.493, 0.49767, 0.502, 0.5095, 0.51233, 0.55167, 0.59833, 0.63633, 0.67633, 0.737, thus ΔA 340 0, 0.01434, 0.01834, 0.024, 0.02567, 0.03034, 0.03467, 0.04217, 0.045, 0.08434, 0.131, 0.169, 0.209, 0.26967, respectively, the concentration of ethyl carbamate (μmol L -1 ) as the abscissa, with the corresponding ΔA 340 As the ordinate, the drawing is the spectrophotometric urethane concentration standard curve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com