A kind of method of reactive extraction separation pantoprazole enantiomer
A technology for pantoprazole and enantiomers, applied in the field of chemical separation engineering, can solve the problem of low selectivity, and achieve the effects of simple process, low cost and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
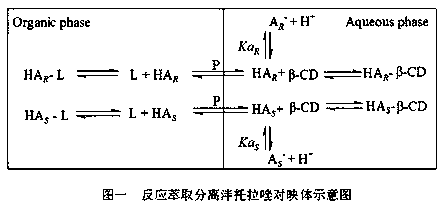
Image
Examples
Embodiment 1
[0015] Dissolve an appropriate amount of R, S-pantoprazole and an appropriate amount of HP-β-CD in 0.1mol / L NaH 2 PO 4 / Na 2 HPO 4 In the buffer solution (pH=8.0), the concentration of the enantiomer of pantoprazole was controlled to be 5 mmol / L, and the concentration of HP-β-CD was 30 mmol / L to obtain an aqueous phase. At the same time, D-isobutyl tartrate, D-n-butyl tartrate, and D-isoamyl tartrate with a concentration of 0.4 mol / L were dissolved in 1,2-dichloroethane respectively, and the organic phase was obtained after thorough stirring. Take 10 mL each of the aqueous phase and the organic phase and put them into test tubes, shake in a constant temperature shaker in a water bath for 9 to 14 hours, and control the constant temperature at 25 °C. Thereafter, the two phases were kept at constant temperature for more than 24 hours. After the two phases are completely separated, the organic phase is extracted, and the water phase is analyzed by HPLC to obtain the concentrat...
Embodiment 2
[0017] Dissolve a certain amount of R,S-pantoprazole in 0.1mol / L NaH 2 PO 4 / Na 2 HPO 4 Buffer solution (pH = 8.5), and then dissolved in a certain concentration of hydroxypropyl-β-cyclodextrin (HP-β-CD), sulfobutyl ether-β-cyclodextrin (SBE-β-CD) , hydroxyethyl-β-cyclodextrin (HE-β-CD) and methyl β-cyclodextrin (Me-β-CD), the concentration of the control pantoprazole enantiomer is 10mmol / L, the ring The concentration of dextrin derivatives is maintained at 60mmol / L to obtain an aqueous phase, while a certain amount of D-isobutyl tartrate is dissolved in 1,2-dichloroethane and the concentration of D-isobutyl tartrate is kept at 0.1mol / L, the organic phase was obtained after thorough stirring. Take 10 mL each of the aqueous phase and the organic phase and put them into test tubes, shake in a constant temperature shaker in a water bath for 9 to 14 hours, and control the constant temperature at 25 °C. Thereafter, the two phases were kept at constant temperature for more tha...
Embodiment 3
[0019] Take appropriate amount of HP-β-CD dissolved in 0.1mol / L NaH 2 PO 4 / Na 2 HPO 4 In buffer solution, pH=6.5, HP-β-CD concentration is 35mmol / L. Control the enantiomeric concentration of pantoprazole in the above-mentioned solution respectively to be 2mmol / L, 4mmol / L, 6mmol / L, 8mmol / L, 10mmol / L, 12mmol / L, obtain six groups of solutions as the water phase. At the same time, a certain amount of isobutyl D-tartrate was dissolved in 1,2-dichloroethane to keep the concentration of isobutyl D-tartrate at 0.25 mol / L, and the organic phase was obtained after thorough stirring. Take 10 mL of the organic phase obtained above and place 10 mL of each of the six groups of water phases into different test tubes, and perform extraction operation in a constant temperature oscillator in a water bath (the constant temperature is controlled at 5°C). Mix well and let stand to separate the layers, so that the two phases reach equilibrium, then detect the extraction effect. The results sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation factor | aaaaa | aaaaa |
| partition coefficient | aaaaa | aaaaa |
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com