Metal complex of chlorophyll degradation product chlorin e6 derivatives as well as preparation method and applications of metal complex
A chlorophyll degradation and metal complex technology is applied in the field of metal complexes of chlorin e6 derivatives, which are chlorophyll degradation products, and their preparation, and can solve the problems of poor stability, limited application, complex components and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
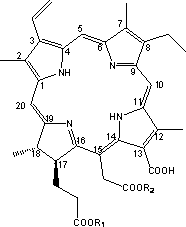
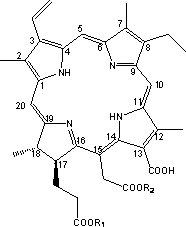
[0039] Example 1: 2,7,12,18-tetramethyl-3-vinyl-8-ethyl-13-carboxy-15-methoxymethyl-17-methoxyethyl-17,18- Preparation of Chlorin
[0040]
[0041] Chlorin e 6 (1.0g, 1.7mmol) was added to methanol (30ml), stirred at room temperature and added dropwise with 98% concentrated sulfuric acid (1ml), and reacted at 55°C for 5h under nitrogen protection. The reaction solution was cooled, diluted with dichloromethane (90ml), poured into ice water (150ml) for extraction, left to stand and separated, the organic phase was concentrated to dryness by rotary evaporation, washed with water and suction filtered, and the filter cake was vacuum-dried and then chromatographed on a silica gel column to obtain 0.75g black fine particle product, yield: 75%. LC-MS (ESI): [M+H] + = 625.
Embodiment 2
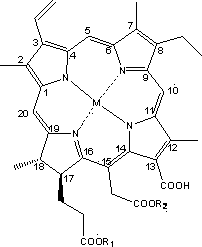
[0042] Example 2: 2,7,12,18-tetramethyl-3-vinyl-8-ethyl-13-carboxy-15-methoxymethyl-17-methoxyethyl-17,18- Preparation of Zinc Complex of Chlorin
[0043]
[0044] 2,7,12,18-tetramethyl-3-vinyl-8-ethyl-13-carboxy-15-methoxymethyl-17-methoxyethyl-17,18-chlorine The dried sample of phenene (1.0g, 1.6mmol) was finely ground, dissolved in dichloromethane (100ml), and zinc acetate (0.5g, 2.3mmol) was completely dissolved in methanol (20ml). 2 Mix well under protection, stir and react at room temperature for 1 h. The reaction solution changed from yellow-brown to blue-green. After the reaction solution was extracted with 2 times the volume of dichloromethane, 5 times the volume of ice water was added and stirred slowly until the organic phase precipitated a solid. After static until liquid separation, the organic phase was suction-filtered, washed with water until the filtrate was clear and transparent, and the filter cake was dried to obtain 0.68 g of black fine particle compo...
Embodiment 3
[0045] Example 3: 2,7,12,18-tetramethyl-3-vinyl-8-ethyl-13-carboxy-15-methoxymethyl-17-methoxyethyl-17,18- Preparation of Copper Complex of Chlorin
[0046]
[0047] 2,7,12,18-tetramethyl-3-vinyl-8-ethyl-13-carboxy-15-methoxymethyl-17-methoxyethyl-17,18-chlorine The dried sample of phenene (1.0g, 1.6mmol) was pulverized, dissolved in 100ml methylene chloride, (0.4g, 2.0mmol) copper acetate was dissolved in 50 ml methanol, and the two were dissolved in N 2 Mix well under protection, and stir the reaction at room temperature for 0.5h. The reaction solution changed from yellowish brown to bright green. After the reaction solution was extracted with 2 times the volume of dichloromethane, 5 times the volume of deionized water was added to slowly stir to wash away free copper ions. After static liquid separation, the lower organic phase was concentrated to dryness by rotary evaporation, soaked in water, stirred and washed several times with water until the filtrate was clear a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com