Method for producing swine erysipelas live vaccine
A production method and technology for vaccine production, applied in bacterial antigen components, antibacterial drugs, etc., can solve the problems of low freeze-drying survival rate, instability between batches, storage, transportation and use difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] - Preparation of synthetic media
[0067] 1. Preparation of Synthetic Medium
[0068] Synthetic medium formula (W / V) is as follows:
[0069] Soytone 10g, Peptone 20g, Yeast Powder 20g, Dipotassium Hydrogen Phosphate 0.675g, Potassium Dihydrogen Phosphate 0.1632g, Magnesium Sulfate 0.2g, Glucose 5g, L-Arg-HCl 5g, Tryptophan 1g, Tween-800.5ml 1. Add water for injection to 1000ml;
[0070] 2. Preparation method of synthetic medium
[0071] According to the above formula, the culture medium components (except glucose) were weighed sequentially and added to the water for injection. After fully dissolving, adjust the pH value to 7.8-8.2 with 2mol / L sodium hydroxide solution, and sterilize at 116°C for 30 minutes. Just before use, aseptically add 50% glucose solution with a final concentration of 0.5%, and mix well.
[0072] 50% glucose solution (W / V) Weigh 50g of glucose, add water for injection to dissolve, dilute to 100ml, sterilize at 116°C for 30 minutes, store at 2-8...
Embodiment 2
[0075] ——Preparation of lyoprotectant
[0076] The formulation of the protectant
[0077] Dextran 10g, BSA 1g, sorbitol 50g, sucrose 50g, hydrolyzed milk protein 5g, L-sodium glutamate 5g, dipotassium hydrogen phosphate 1.25g, potassium dihydrogen phosphate 0.52g, water for injection 1000ml.
[0078] 2. Preparation method
[0079] Solution 1: In a clean beaker, add an appropriate amount of water for injection (100°C), weigh dextran, BSA, and sorbitol in turn according to the formula, add them to the beaker, and stir with a glass rod while adding to make it completely dissolve, then use Dilute to 500ml with water for injection, and sterilize at 116°C for 30 minutes.
[0080] Solution 2: Weigh sucrose, hydrolyzed milk protein, sodium L-glutamate, potassium dihydrogen phosphate and dipotassium hydrogen phosphate in a beaker in turn according to the formula, add water for injection to fully dissolve it, and set the volume to 500ml, 0.22μm Membrane-sterilizing filters are steril...
Embodiment 3
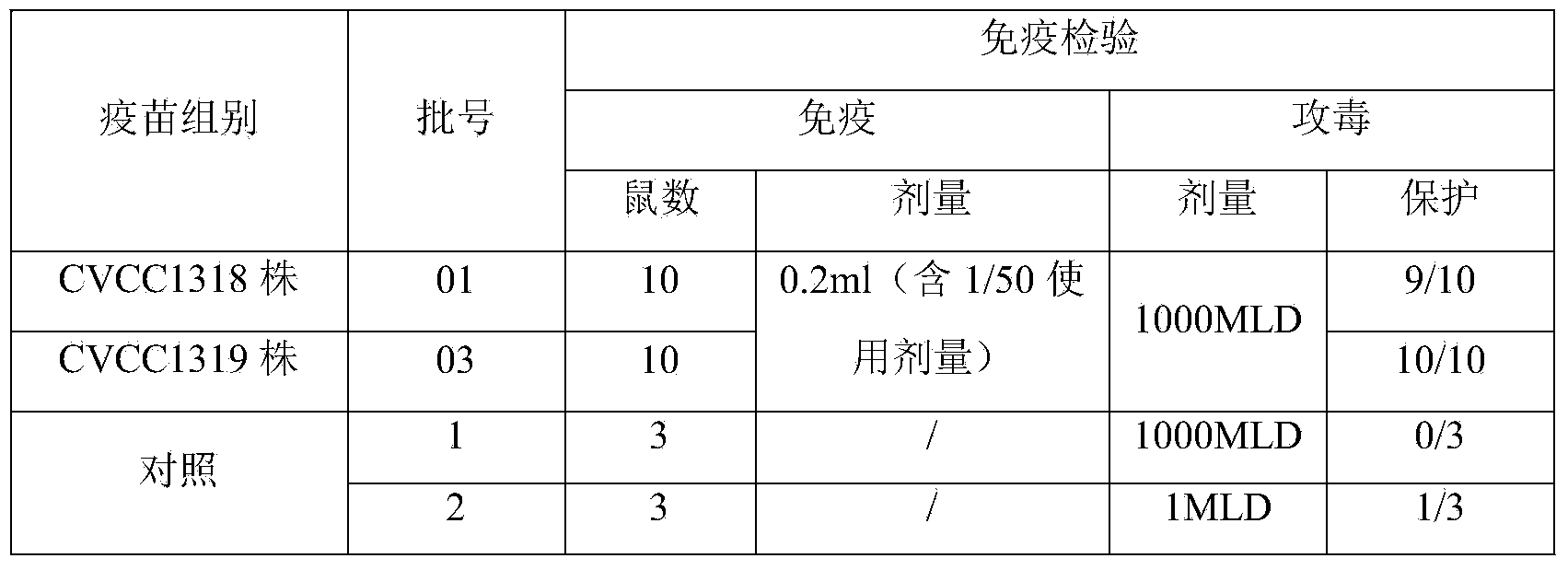
[0083] ——Erysipelas live vaccine (CVCC1318)
[0084] 1 Preparation of bacterial solution for seedling production
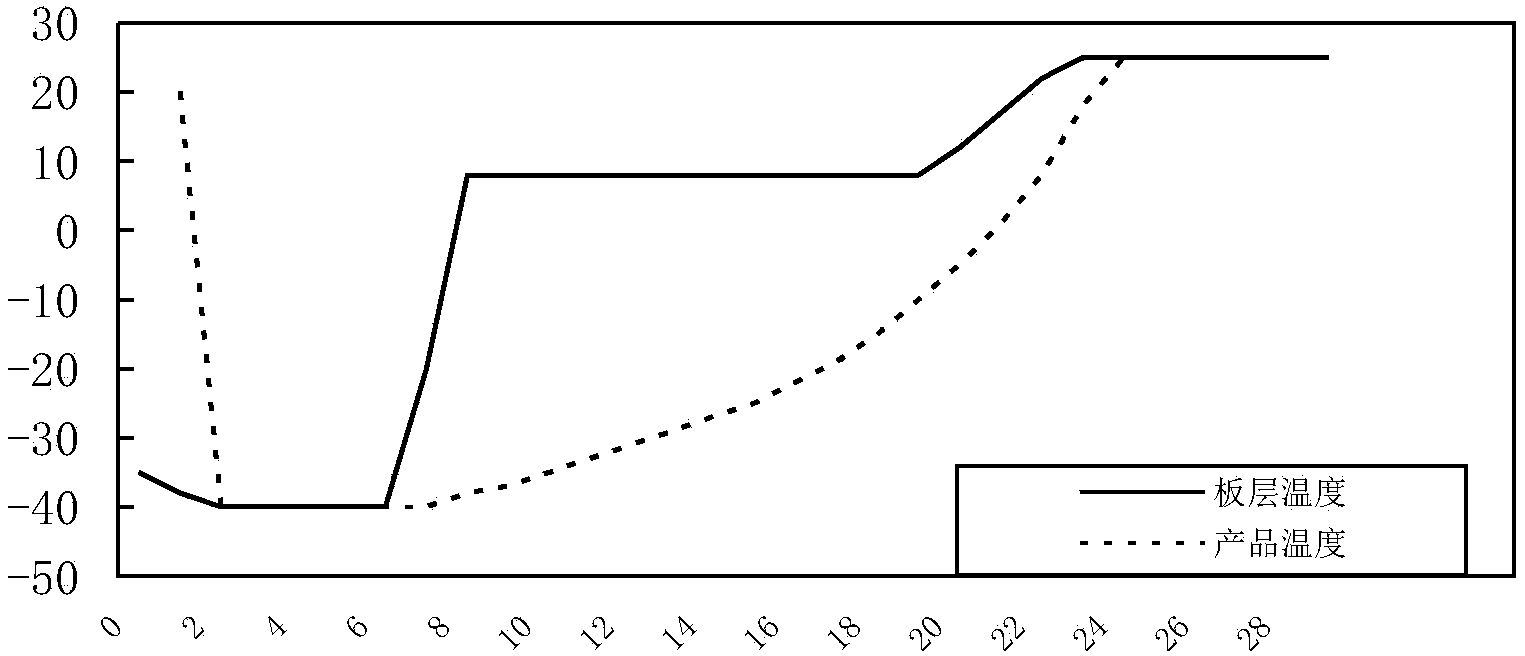
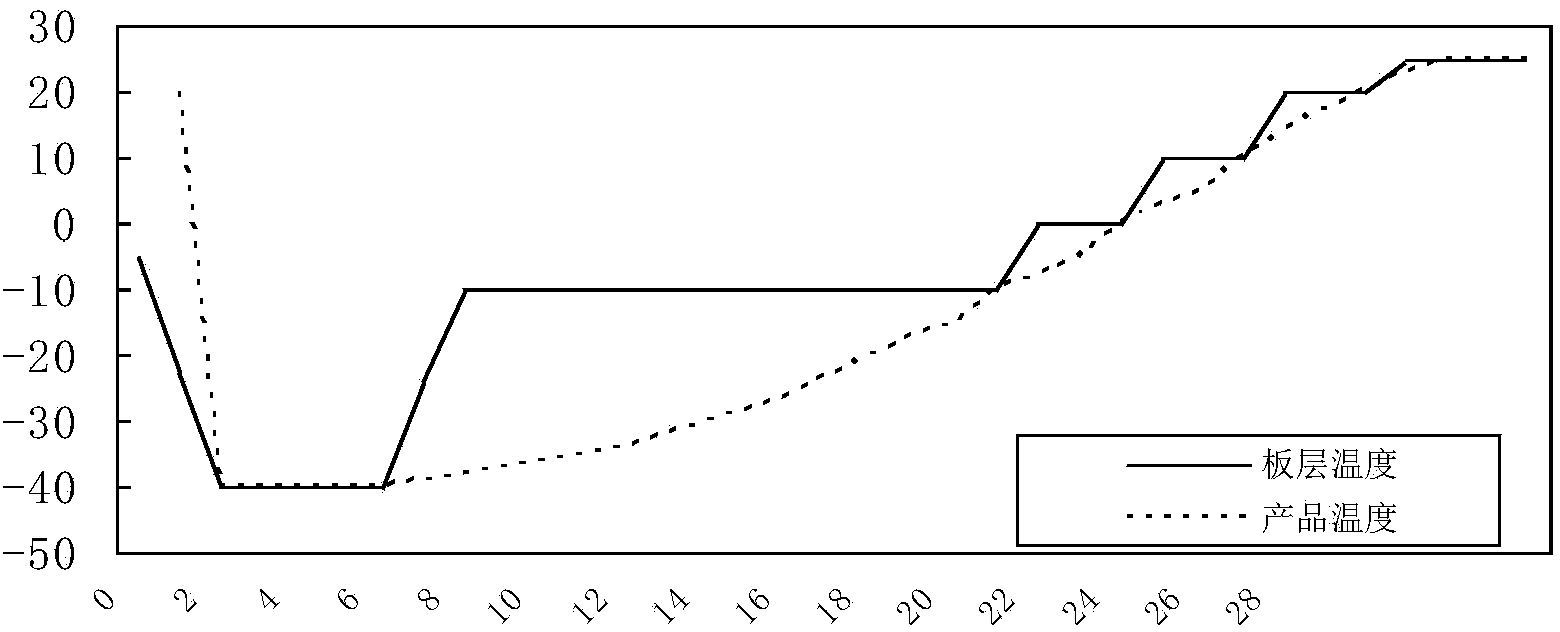
[0085] Inoculate the Erysipelas suis CVCC1318 seed liquid into the liquid medium according to 1% to 2% of the total medium, ferment or aerate at 37°C for 8 to 10 hours, add an appropriate amount of antifoaming agent according to the need during the cultivation process, and When the pH rises, add an appropriate amount of sterilized 2mol / L sodium hydroxide solution to control the pH value. After the cultivation, the bacterial solution was centrifuged at 3500r / min for 20min, and the bacterial precipitate was harvested. The precipitate was suspended by adding an appropriate amount of heat-resistant freeze-drying protective agent that had been sterilized and preheated to 37°C. During the subpackaging process, attention should be paid to keeping warm and shaking evenly. After subpackaging, freeze and vacuum dry quickly, according to the method stipulated in the "Chines...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com