Preparation method of polycaprolactone/polyethylene glycol hydrogel used for photodynamic therapy
A technology of photodynamic therapy and polycaprolactone, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., to achieve easy-to-operate and reversible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
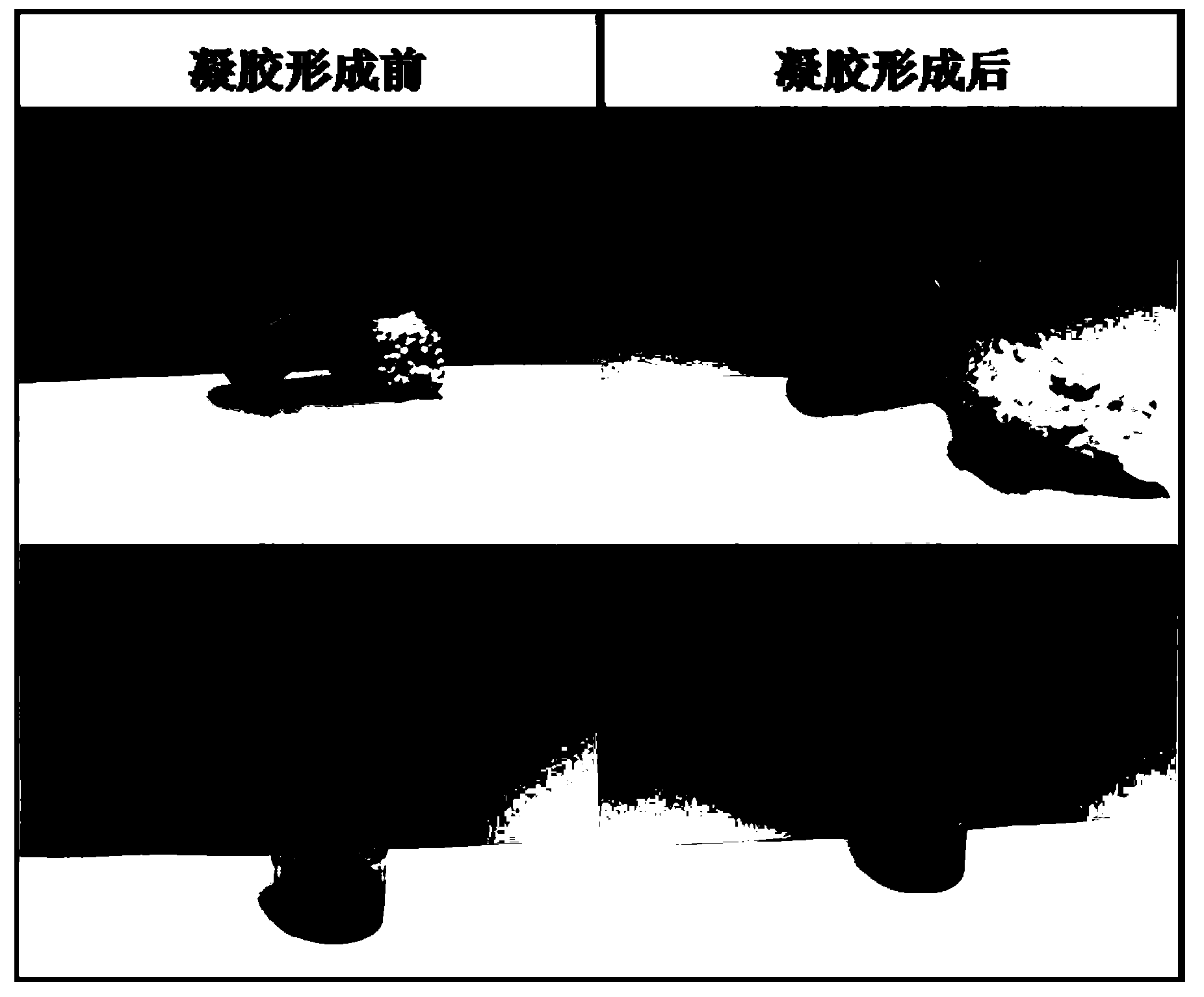
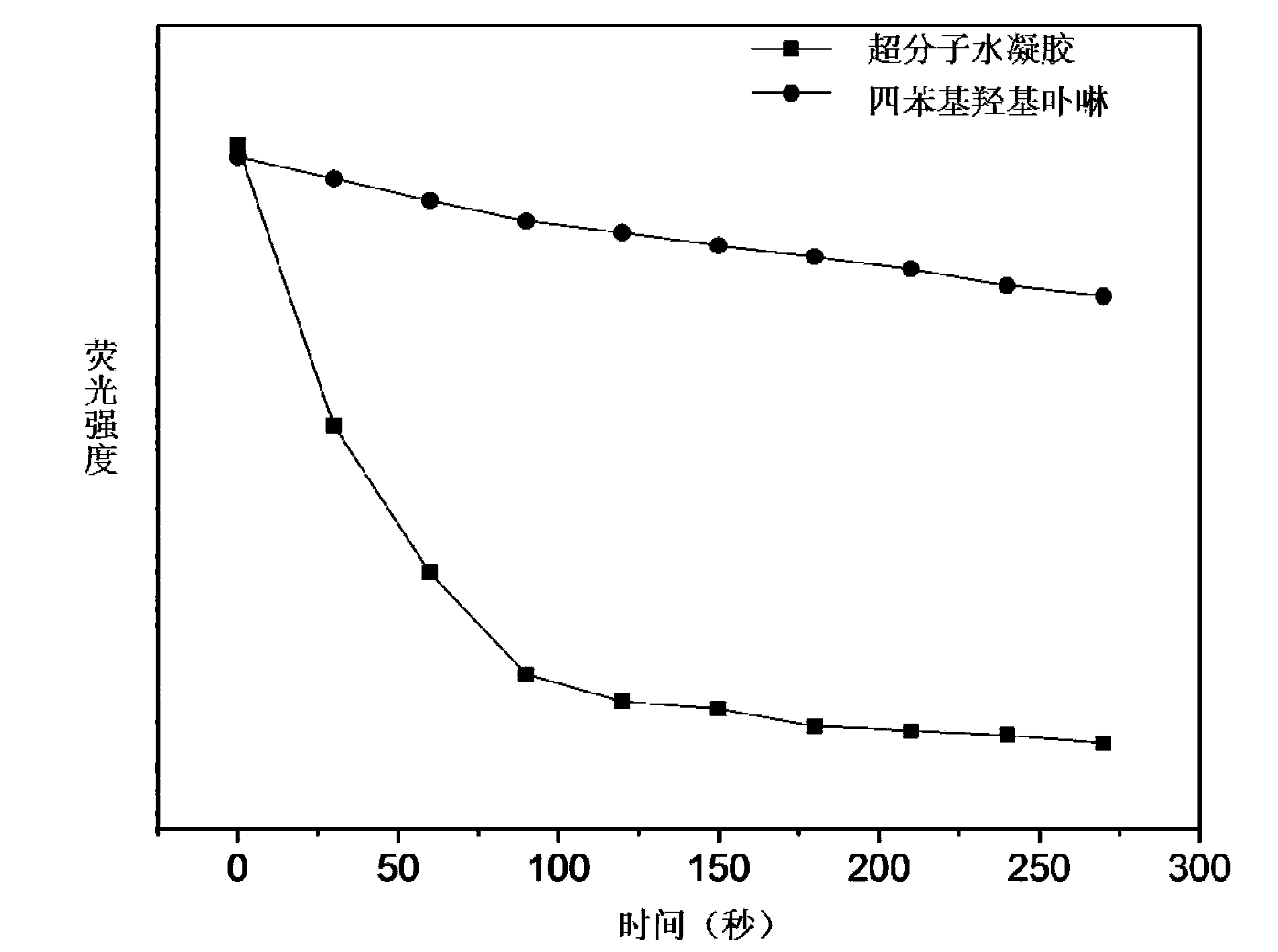
Image
Examples
Embodiment 1
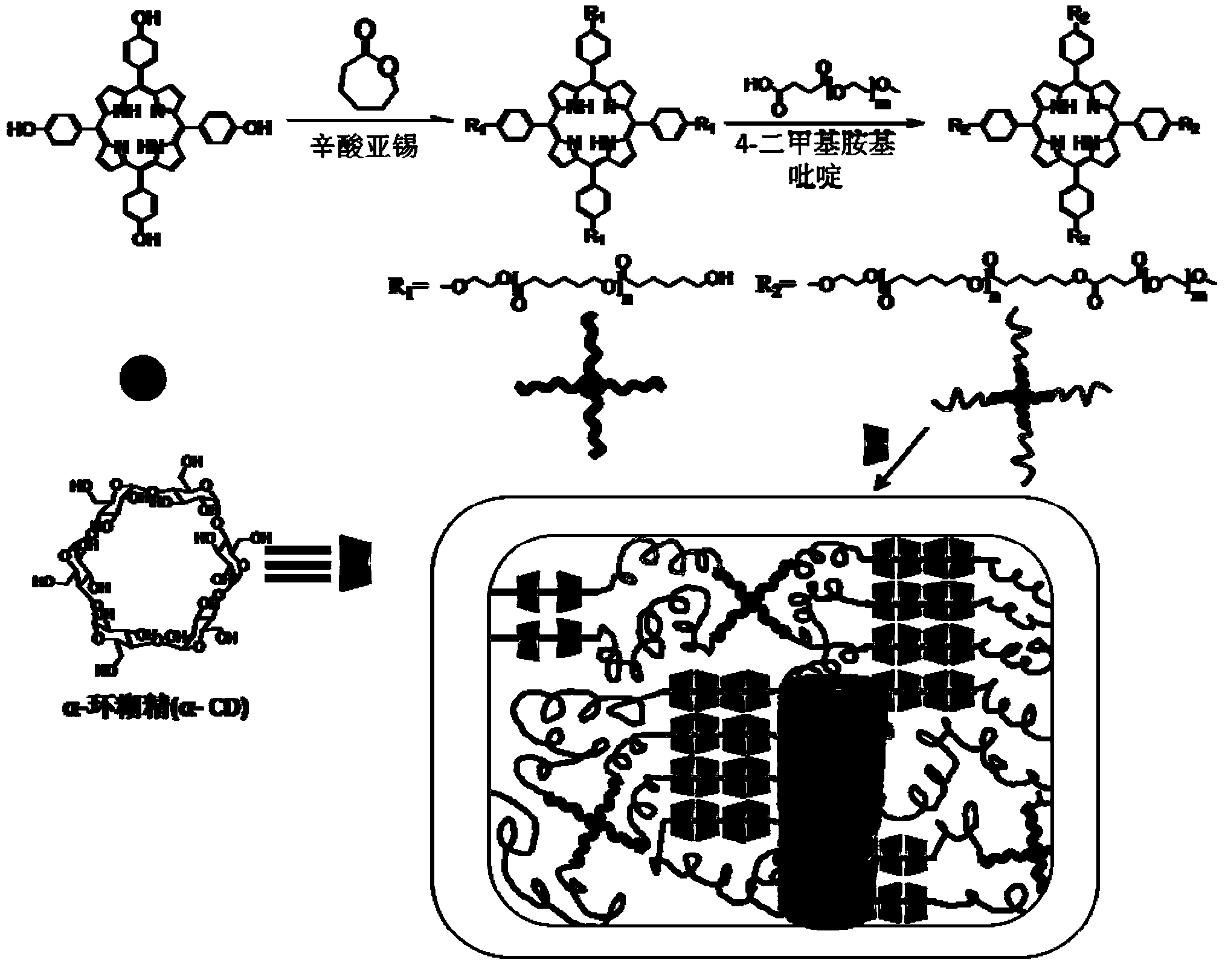
[0019] Embodiment 1: Preparation method of star polycaprolactone-block-polyethylene glycol biosupramolecular hydrogel with porphyrin as core
[0020] p-5,10,15,20-Tetrakis(2-hydroxyethyl)phenylporphyrin as initiator (57.8mg, 0.075mmol), caprolactone (300mg, 3mmol) were placed in fully dried test tubes , sealed with a turn-over plug, operated on the vacuum line, evacuated and ventilated with nitrogen for three times, then put in 120 o C In a constant temperature oil bath, stannous octoate (5.5 mg) was added into the micro-injector. After reacting for 24 hours, the test tube was cooled to room temperature, and the obtained solid was dissolved in dichloromethane, and settled dropwise in ice methanol under magnetic stirring. at 30 o C under vacuum constant temperature drying to constant weight, to obtain polycaprolactone (289mg, M n =4800).
[0021] Add polycaprolactone (M n =4800, 192mg, 0.004mmol), 1.1-fold excess carboxylated polyethylene glycol (M n =5000, 82.5mg, 0.0165...
Embodiment 2
[0024] Embodiment 2: The preparation method of star-shaped polycaprolactone-block-polyethylene glycol biosupramolecular hydrogel with porphyrin as the core
[0025] p-5,10,15,20-tetrakis(2-hydroxyethyl)phenylporphyrin as initiator (38.5mg, 0.05mmol), caprolactone (300mg, 3mmol) were placed in fully dried test tubes , sealed with a turn-over plug, operated on the vacuum line, evacuated and ventilated with nitrogen for three times, then put in 120 o In the C constant temperature oil bath, the micro-injector adds stannous octoate (SnOct 2) (5.5mg). After reacting for 24 hours, the test tube was cooled to room temperature, and the obtained solid was dissolved in dichloromethane, and settled dropwise in ice methanol under magnetic stirring. at 30 o C under vacuum constant temperature drying to constant weight, to obtain polycaprolactone (253mg, M n =6800).
[0026] Add polycaprolactone (M n =6800, 272mg, 0.004mmol), 1.1-fold excess carboxylated polyethylene glycol (M n =5000...
Embodiment 3
[0029] Embodiment 3: The preparation method of star polycaprolactone-block-polyethylene glycol biosupramolecular hydrogel with porphyrin as core
[0030] p-5,10,15,20-Tetrakis(2-hydroxyethyl)phenylporphyrin as initiator (57.8mg, 0.075mmol), caprolactone (300mg, 3mmol) were placed in fully dried test tubes , sealed with a turn-over plug, operated on the vacuum line, evacuated and ventilated with nitrogen for three times, then put in 120 o C In a constant temperature oil bath, stannous octoate (5.5 mg) was added into the micro-injector. After reacting for 24 hours, the test tube was cooled to room temperature, and the obtained solid was dissolved in dichloromethane, and settled dropwise in ice methanol under magnetic stirring. at 30 o C under vacuum constant temperature drying to constant weight, to obtain polycaprolactone (289mg, M n =4800).
[0031] Add polycaprolactone (M n =4800, 192mg, 0.004mmol), 1.1-fold excess carboxylated polyethylene glycol (M n =5000, 82.5mg, 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com