Preparation technology and application of natural recombinant nanostructured lipid carrier
A natural and lipid technology, applied in drug combination, freeze-dried delivery, powder delivery, etc., can solve the problems of total protein miscellaneous, unfavorable waste utilization, low economic value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Extraction and purification process of natural apolipoprotein and lipid in plasma fraction IV
[0042] The invention provides a natural apolipoprotein extraction and purification process, comprising the following steps:
[0043] Utilize organic solvent combined with isoelectric point precipitation method, the steps of extracting apolipoprotein mixture from human plasma fraction IV are as follows:
[0044] Step 1) Weigh plasma fraction IV, initially dissolve in 50ml of phosphate buffer solution with pH 5.25, add 16.6ml of 25% ethanol solution to continue dissolving for an appropriate time, and centrifuge at 12000r / min for 15min.
[0045] Step 2) Take the precipitate, initially dissolve it in 50ml of phosphate buffer solution with pH 6.6, add 4.4ml of 8% ethanol solution to continue dissolving for an appropriate time, and centrifuge at 12000r / min for 15min.
[0046] Step 3) Take the supernatant, adjust the pH to 5.25 with dilute hydrochloric acid, add 12.5ml o...
Embodiment 2
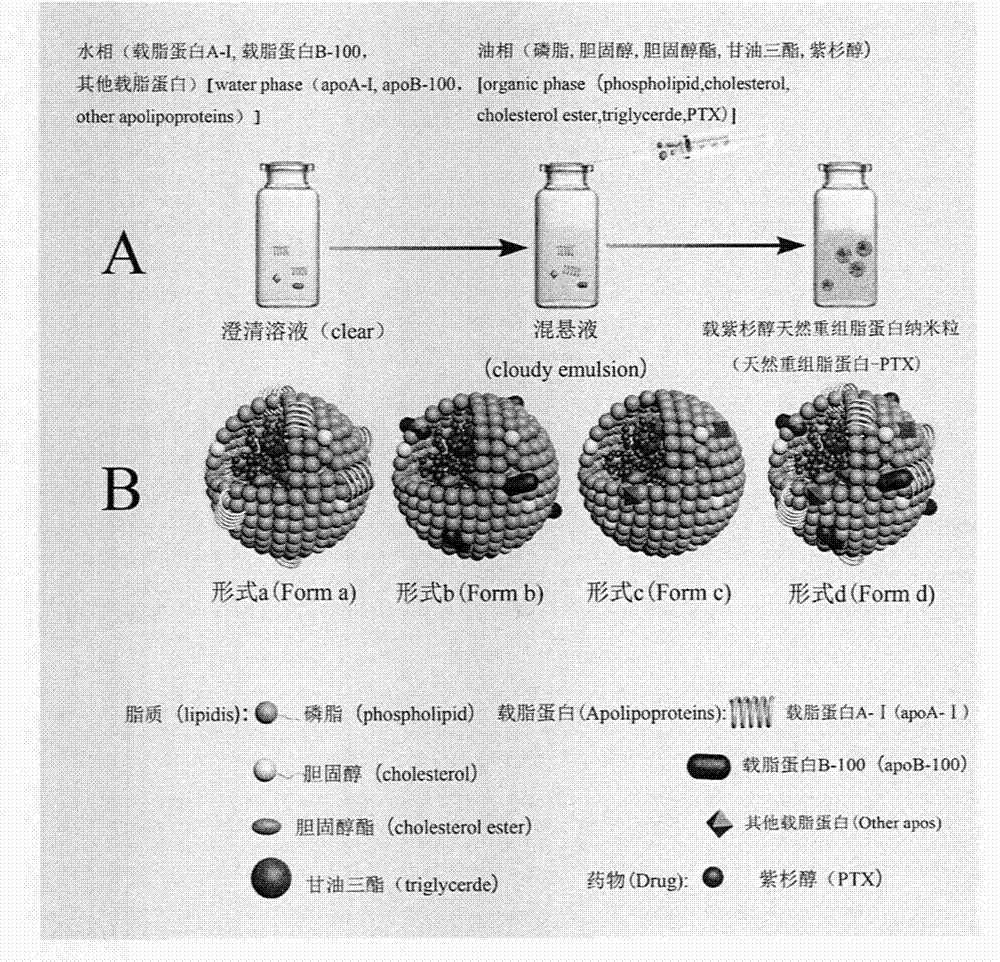
[0050] Embodiment two: the preparation process of carrying PTX natural recombinant lipoprotein (natural recombinant lipoprotein-PTX) nanoparticle
[0051] 1.1 Preparation of natural recombinant lipoprotein-PTX nanoparticles by emulsification evaporation method
[0052]
[0053] The natural lipoprotein mixture and lipids were obtained by organic solvent combined with isoelectric point precipitation method, and the natural lipid ethanol solution dissolved in PTX was slowly added drop by drop with a syringe under the action of magnetic stirring at room temperature to the phosphate buffer solution of apolipoprotein , after the dropwise addition, continue magnetic stirring for 50 min; remove ethanol by rotary evaporation at 37°C, and conduct probe ultrasound under ice bath conditions, pass through a 0.22 μm filter membrane, and freeze-dry to obtain natural recombinant lipoprotein-PTX nanoparticles.
[0054] 1.2 Preparation of natural recombinant lipoprotein-PTX nanoparticles by ...
Embodiment 3
[0060] Example 3: Research on the properties of natural recombinant lipoproteins
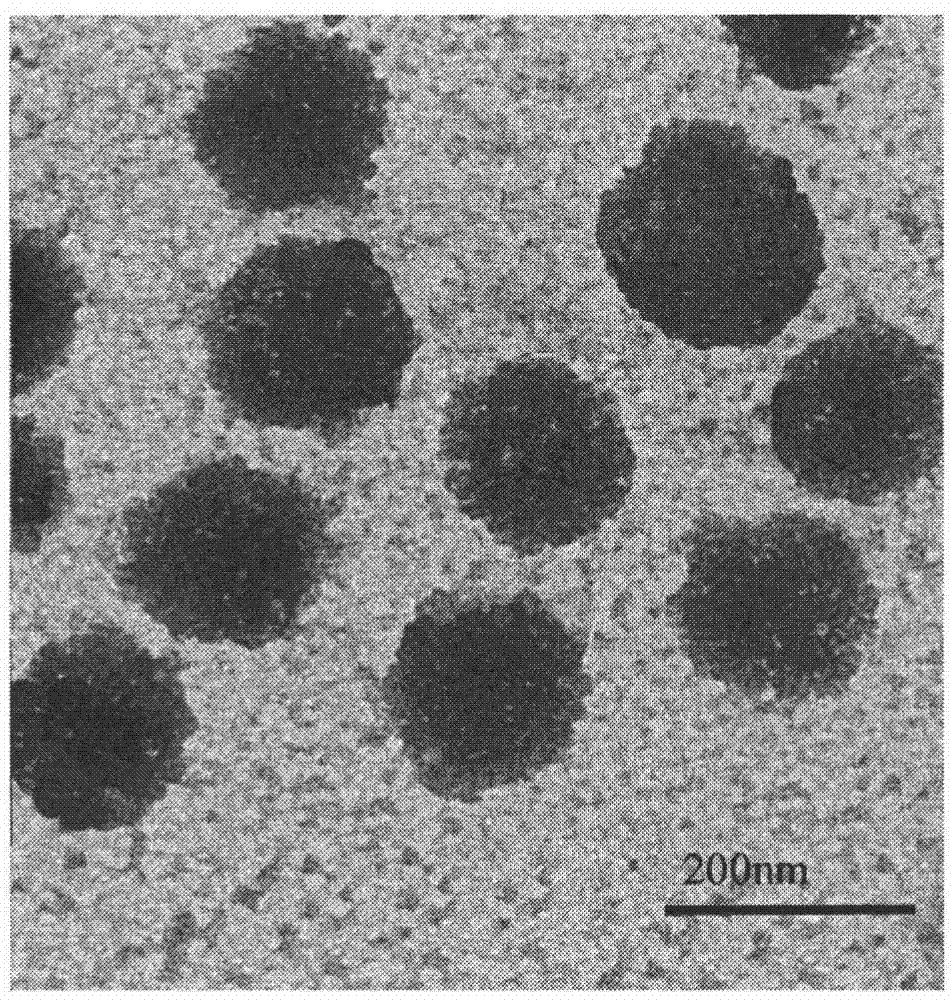
[0061] 2.1 Particle size and morphology of natural recombinant lipoprotein-PTX nanoparticles
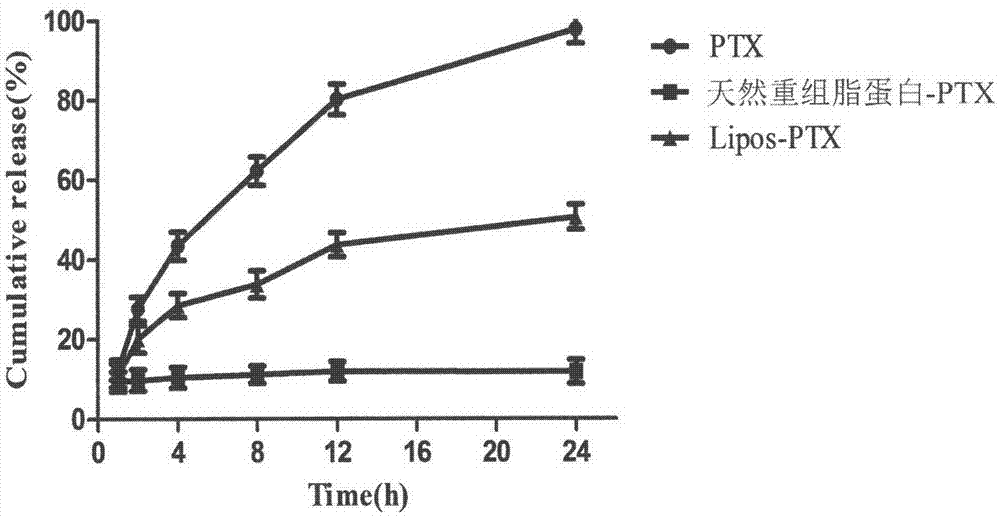
[0062] The average particle size of natural recombinant lipoprotein-PTX nanoparticles was measured by laser particle size analyzer (160.4±20)nm. Transmission electron microscope results are as figure 1 As shown, the prepared natural recombinant lipoprotein-PTX nanoparticles are all spherical particles with good roundness, smooth appearance, and consistent with the particle size measured by the laser particle size analyzer.
[0063] 2.2 Encapsulation efficiency of PTX in natural recombinant lipoprotein-PTX nanoparticles
[0064] Take 100 μl of natural recombinant lipoprotein-PTX nanoparticles, dilute to 5ml with methanol, sonicate in a water bath for 20 minutes, centrifuge at 12000r / min for 10 minutes, take the supernatant and filter it with a 0.22 μm organic filter membrane, and measure its content b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com