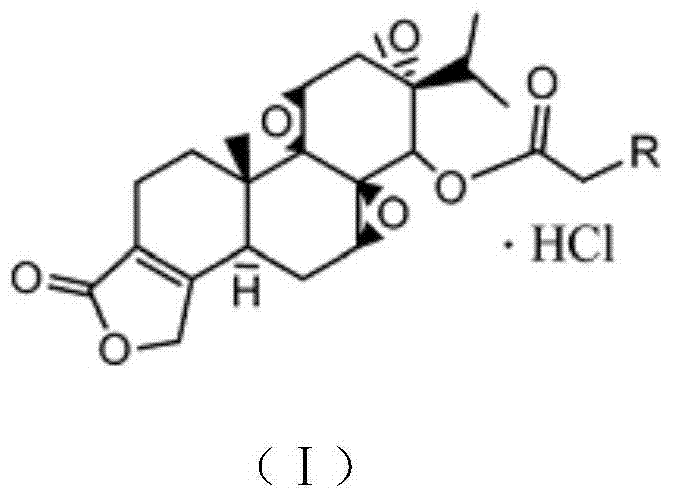
Triptolide derivatives and application thereof
A technology for triptolide and derivatives, which is applied to triptolide derivatives and their application fields, and can solve the problems of poor water solubility, high toxicity, and low target specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
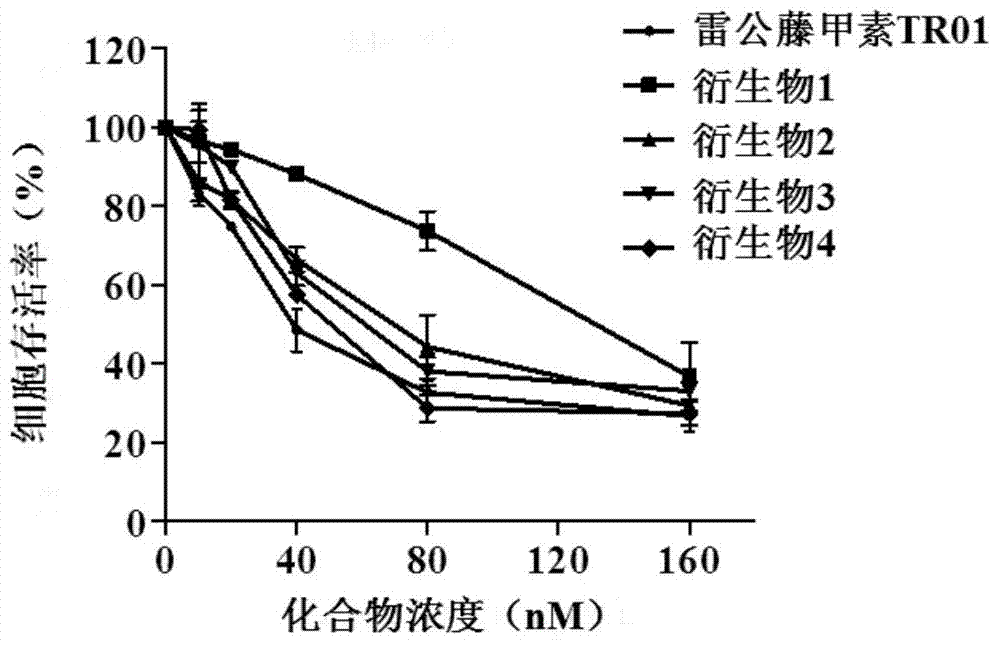
[0021] Example 1: Dose curve and IC of triptolide and its derivatives inhibiting cell viability 50 value determination
[0022] In this embodiment, the MTT method is used to measure the influence of triptolide derivatives on the proliferation of various cancer cells. The specific operations are as follows:
[0023] (1) Experimental materials
[0024] Cell lines: HepG2 (ATCC HB-8065), MCF-7 (ATCC HTB-22).
[0025] Cancer cell lines were cultured with DMEM (Gibco) + 10% fetal bovine serum (Hyclone). The experiment also needs triptolide and its derivatives (self-synthesized), 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (Sigma).
[0026] (2) Experimental method
[0027] The MTT method was used to detect that triptolide and its derivatives have an inhibitory effect on cell proliferation, and the specific determination method is as follows:
[0028] A. Digest the logarithmic phase cells with trypsin, collect by centrifugation after termination, make cell suspensi...
Embodiment 2
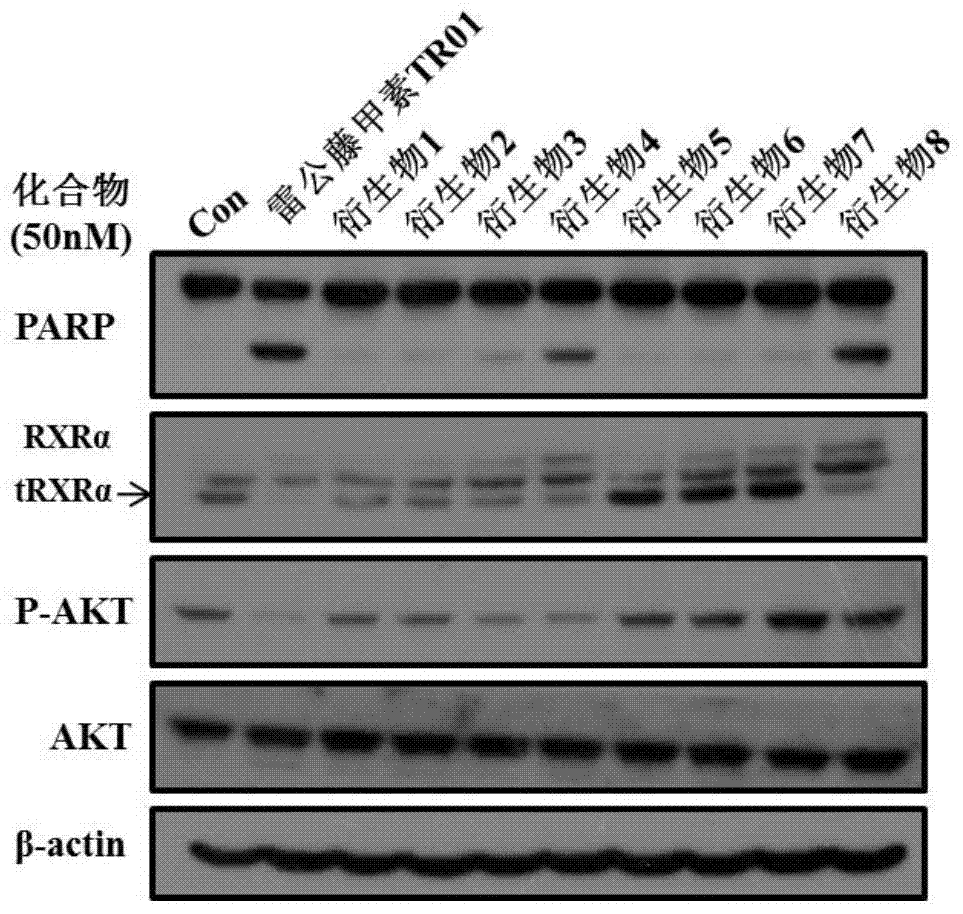
[0040] Example 2: Detection of PARP cleavage, RXRα and tRXRα degradation induced by triptolide and its derivatives by Western blot
[0041]Select liver cancer cells HepG2, and culture them in 6-well tissue culture plates at 37°C and 5% carbon dioxide incubator, with 50nM triptolide (TR01) and its derivatives (No. 1, No. 2, No. 3, No. 4, No. 5, No. 6 and No. 7), serum-free DMEM were treated for 24h. Then, it was lysed on ice for 30 min with improved RIPA lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 5 mM EDTA; 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS). With the same loading amount, 8% SDS-PAGE electrophoresis, transfer membrane, block with 5% skimmed milk powder TBST (50mM Tris-HCl (pH 7.4), 150mM NaCl and 0.1% Tween-20) at room temperature for 1h, at 4°C Incubate the primary antibody overnight, incubate the secondary antibody at room temperature for 1 h, develop color with ECL, and expose. Finally, PARP, RXRα, tRXRα and β-actin were detected.
[0042] Experimental ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com