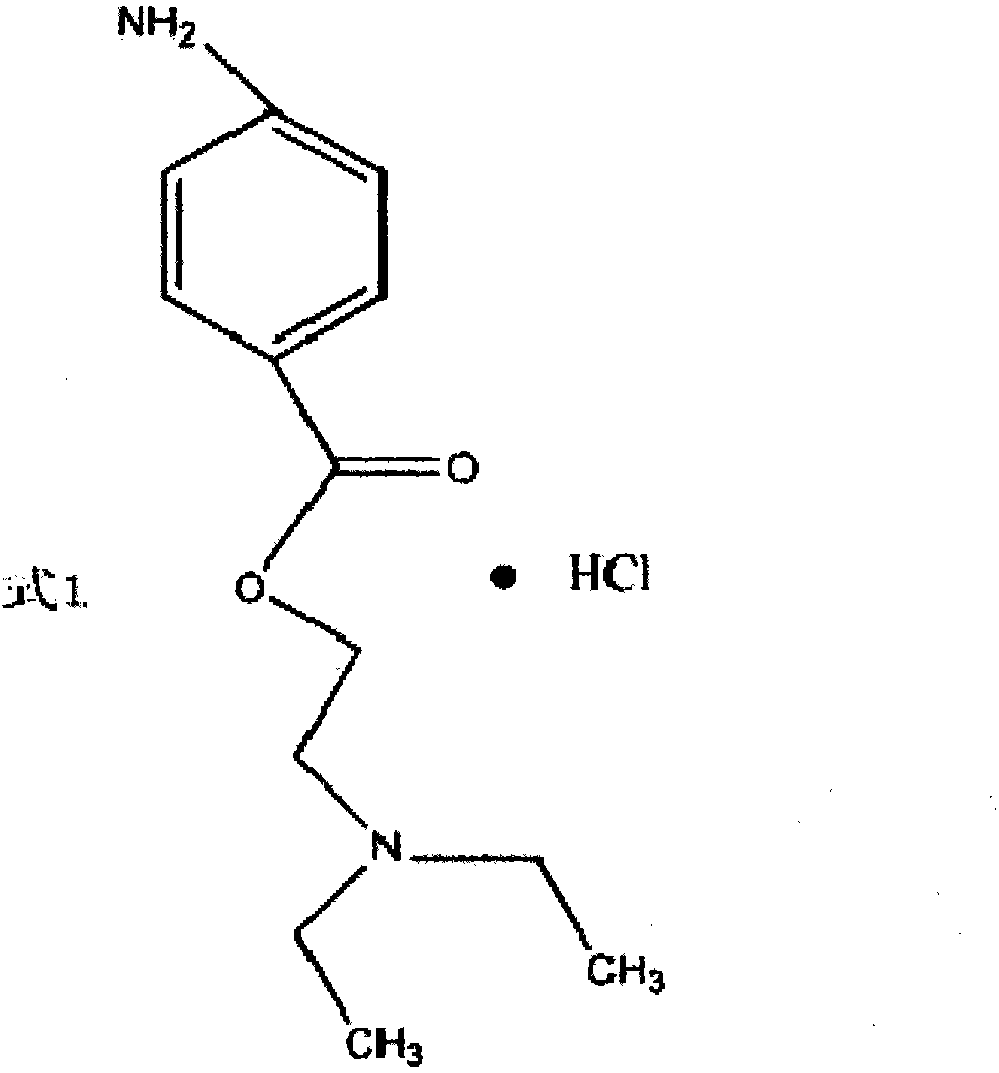
A synthetic method of procaine hydrochloride
A technology of procaine hydrochloride and a synthesis method, which is applied in the field of synthesis of pharmaceutical compounds, can solve the problems of low yield, complicated operation procedures, low yield of procaine hydrochloride, etc., and achieves an increase in synthesis rate and wide application. Basic, reducing the effect of iron removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 60g of p-nitrobenzoic acid, 360g of xylene, and 44g of diethylaminoethanol into the three-necked flask, heat to reflux to separate water, the reaction temperature is 141-143°C, and react for 12 hours; after the reaction is completed, cool to below 20°C and depressurize Recover xylene; after recovery, cool to below 40°C, slowly add 570g of 6% dilute hydrochloric acid dropwise, after adding, stir for 30 minutes, cool to 20-25°C, filter to obtain filtrate;
[0043]Put the filtrate from the previous step into the hydrogenation reaction kettle, add 15g of catalyst Ni powder, replace it with nitrogen twice, pass in hydrogen, the pressure is 10 standard atmospheres, control the reaction temperature at 50-60°C to carry out the hydrogenation reaction, after the reaction is completed, filter, and in Add 16g of refined salt to the filtrate, stir to dissolve, and cool to 10°C to obtain the product.
Embodiment 2
[0045] Add 600g of p-nitrobenzoic acid, 3600g of xylene, and 440g of diethylaminoethanol into a three-necked flask, heat to reflux to separate water, and react for 24 hours at a reaction temperature of 141-143°C; after the reaction is completed, cool to below 20°C and depressurize Recover xylene; after recovery, cool to below 40°C, slowly add 5700g of 3% dilute hydrochloric acid dropwise, after adding, stir for 20 minutes, cool to 20-25°C, filter to obtain filtrate;
[0046] Put the filtrate from the previous step into the hydrogenation reaction kettle, add 300g of catalyst Pb / C (the mass ratio of the two is 1:3), replace it with nitrogen twice, feed hydrogen, the pressure is 20 standard atmospheres, and the reaction temperature is controlled at 100-120°C Carry out the hydrogenation reaction, after the reaction is completed, filter, add 160 g of refined salt to the filtrate, stir to dissolve, and cool to 10°C to obtain the product.
Embodiment 3
[0048] Add 60g of p-nitrobenzoic acid, 360g of xylene, and 44g of diethylaminoethanol into the three-necked flask, heat to reflux to separate water, the reaction temperature is 141-143°C, and react for 8 hours; after the reaction is completed, cool to below 20°C and depressurize Recover xylene; after recovery, cool to below 40°C, slowly add 570g of 8% dilute hydrochloric acid dropwise, after adding, stir for 60 minutes, cool to 20-25°C, filter to obtain filtrate;
[0049] Add the filtrate from the previous step into the hydrogenation reactor, and add the catalyst Pb / Al 2 o 3 (The mass ratio of the two is 1: 2) 5g, replace 2 times with nitrogen, pass into hydrogen, the pressure is 3 standard atmospheres, control the reaction temperature 30-40 ℃ to carry out hydrogenation reaction, after the reaction is completed, filter, add refined salt 16g in the filtrate , Stir to dissolve, cool to 10 ° C, that is, too.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com