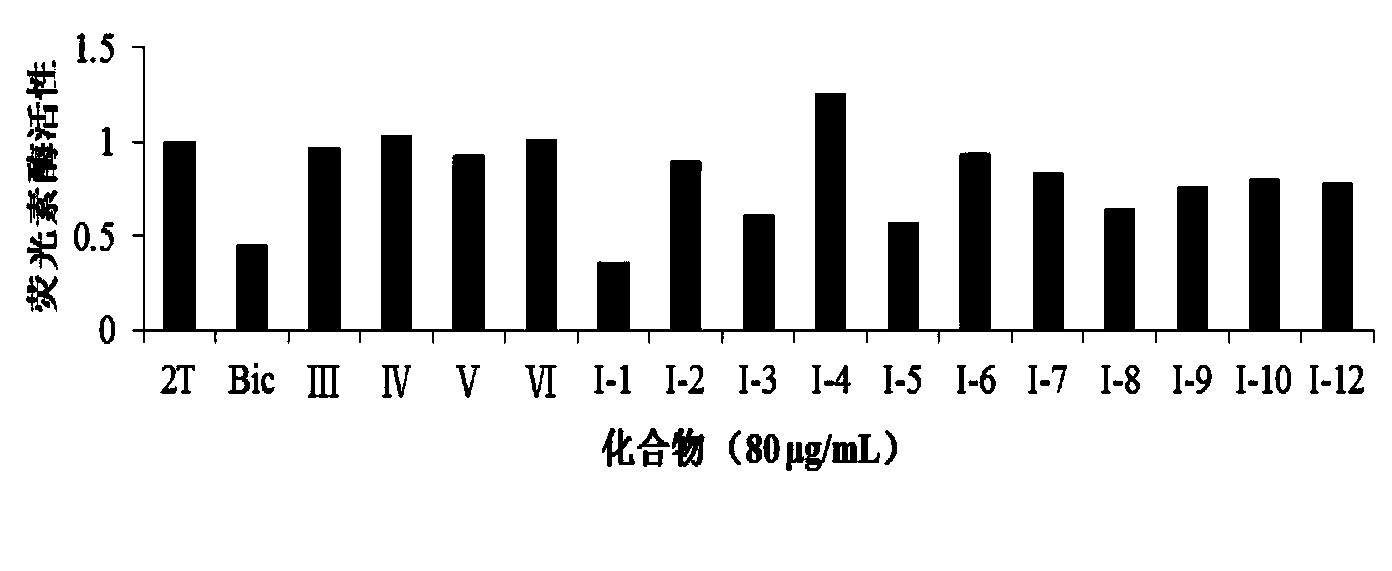
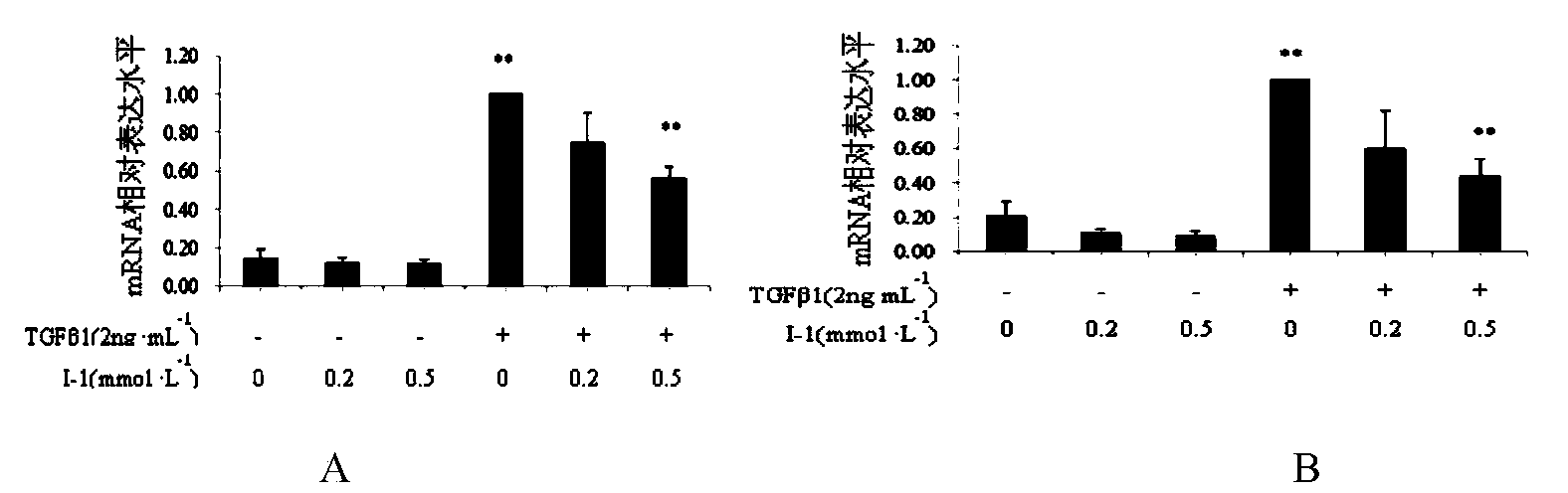
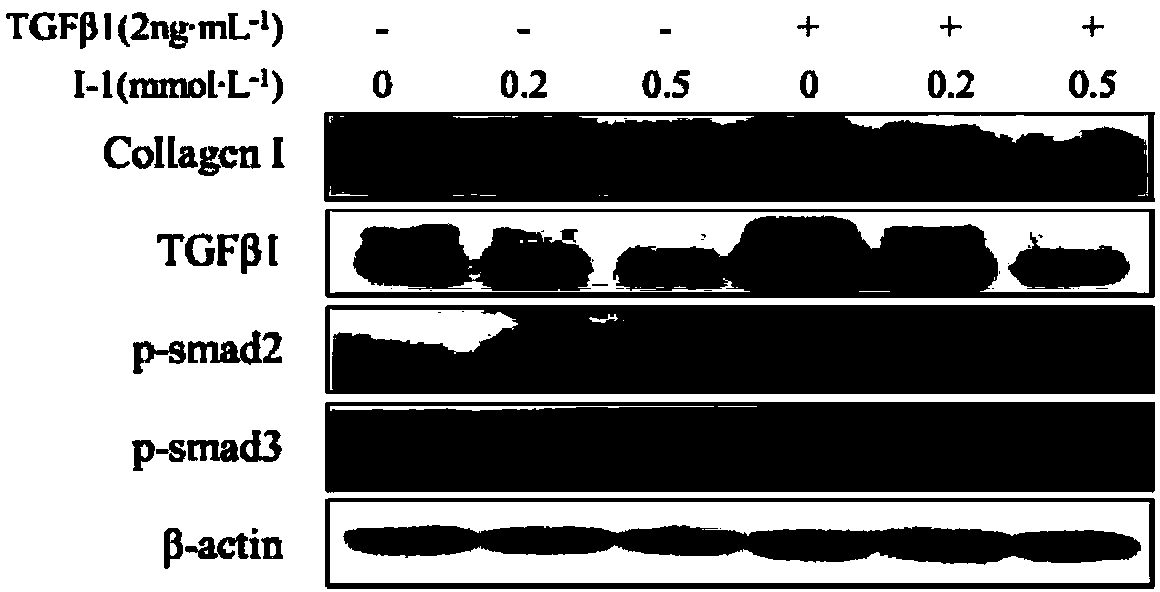
Novel 2'-carbamyl biphenyl compounds and application thereof in anti-hepatic fibrosis medicines
A carbamoyl biphenyl, anti-liver fibrosis technology, applied in the directions of effective components of heterocyclic compounds, digestive system, organic chemistry, etc., to achieve the effects of simplifying the process, improving the yield, and facilitating large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] "Example 1" Synthesis of 4,4'-dimethoxy-5,6,5',6'-dimethoxy-2,2'-dicarboxylic acid biphenyl (III)
[0030] Add 4,4'-dimethoxy-5,6,5',6'-dimethoxy-2,2'-dicarboxylic acid methyl biphenyl (II) (500g, 1.20 mol), add acetone (5000 mL) and water (3000 mL), stir, the material cannot be completely dissolved, then add sodium hydroxide (250g, 6.25 mol), stir and heat to reflux, reflux for about 0.5h, the reaction system is light yellow and clear liquid, refluxed for 3-4 hours, the raw materials basically reacted completely (TLC detection), after the reaction was completed, the acetone was evaporated under reduced pressure, the temperature of the reaction bottle was lowered, and concentrated hydrochloric acid was added under ice bath to acidify to slightly acidic (pH=1-2), and a large amount of white Solid, stirred at room temperature for 0.5h, filtered with suction, washed with water 3-4 times close to neutral, and dried in vacuum at 40°C to obtain 441.4g of white solid product 4...
Embodiment 2
[0032] 《Example 2》Synthesis of acid anhydride derivatives (IV)
[0033] Add 4,4'-dimethoxy-5,6,5',6'-dimethoxy-2,2'-dicarboxylic acid biphenyl (III) (619g, 1.58mol) into a 10 L reaction flask , add acetic anhydride (4300 mL), reflux for 3 hours, distill off acetic anhydride under reduced pressure, add toluene (1000 mL) and petroleum ether (3000 mL) to the residue, stir and filter with suction to obtain a crude product, which is heated with toluene (1500 mL) Stir and wash, cool to room temperature and filter to obtain 516.2g off-white solid anhydride derivative (IV), melting point: 265-266°C, yield: 98.0%.
[0034] 1 H-NMR (400 MHz, CDCl 3 )δ: 7.05(s,2H), 6.06-6.17(m,4H), 3.96(s,6H).
Embodiment 3
[0035] "Example 3" Synthesis of 4,4'-dimethoxy-5,6,5',6'-dimethoxy-2-formic acid-2'-hydroxymethylbiphenyl (V)
[0036] Add an acid anhydride derivative (IV) (446.7g, 1.2mol) into a 10 L reaction flask, add tetrahydrofuran (THF 6000mL), add sodium borohydride (150.0g, 3.96 mol) in batches at room temperature, after the addition is complete, stir at room temperature for 8 h , slowly added 6 mol / L hydrochloric acid to the reaction solution until no bubbles were produced, evaporated THF, added 1000 mL of water to dissolve the residue, extracted with ethyl acetate, washed with water, dried, and concentrated to obtain 411.5 g of off-white solid product 4 , 4′-dimethoxy-5,6,5′,6′-dimethoxy-2-formic acid-2′-hydroxymethylbiphenyl (V), yield: 91.7%.
[0037] 1 H-NMR (400 MHz, CDCl 3)δ:7.40(s,1H),6.74(s,1H),6.02-6.05(m,2H),5.91-5.92(m,2H),4.35-4.44(m,2H),3.97(s, 3H), 3.94(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com