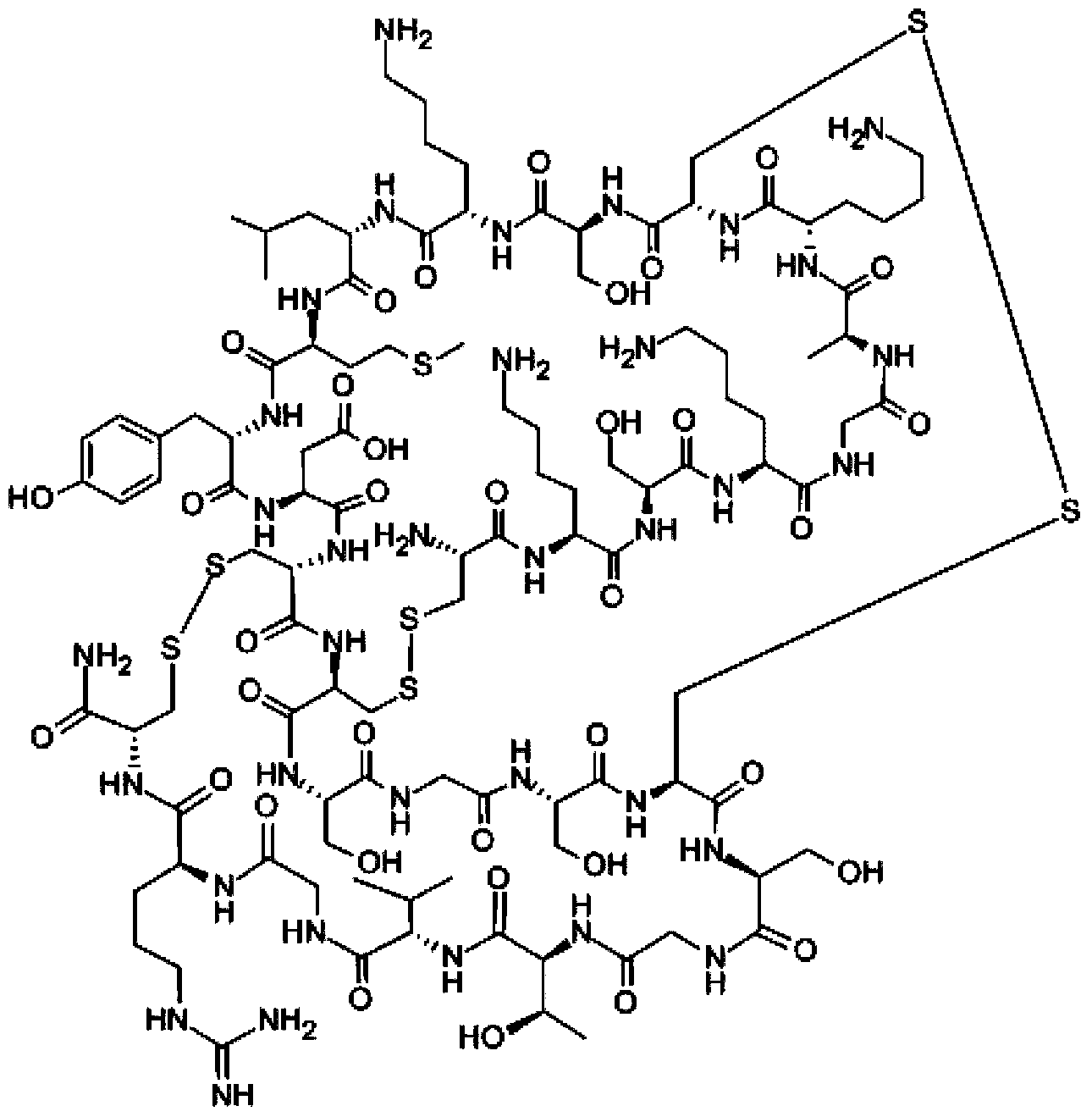
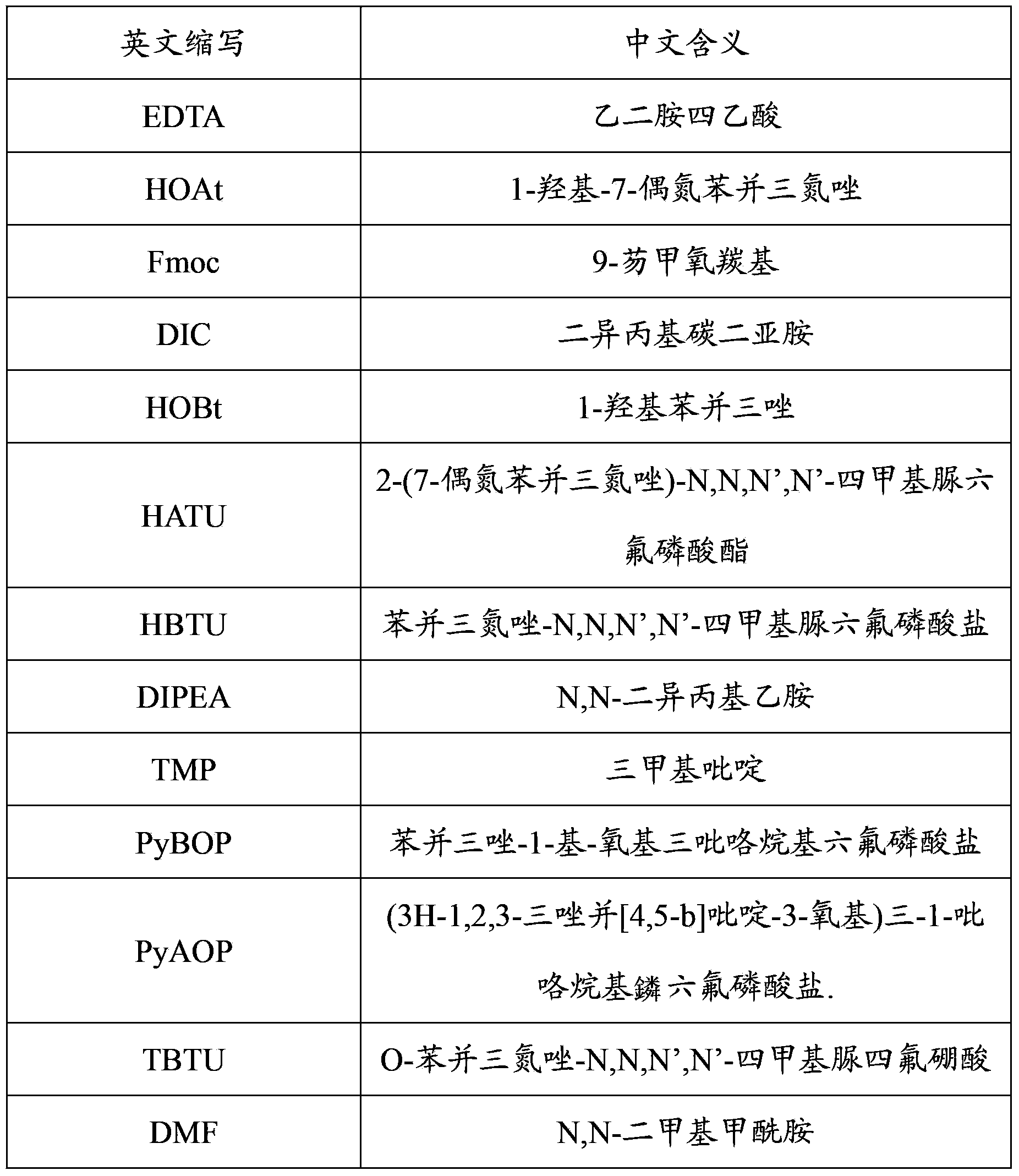
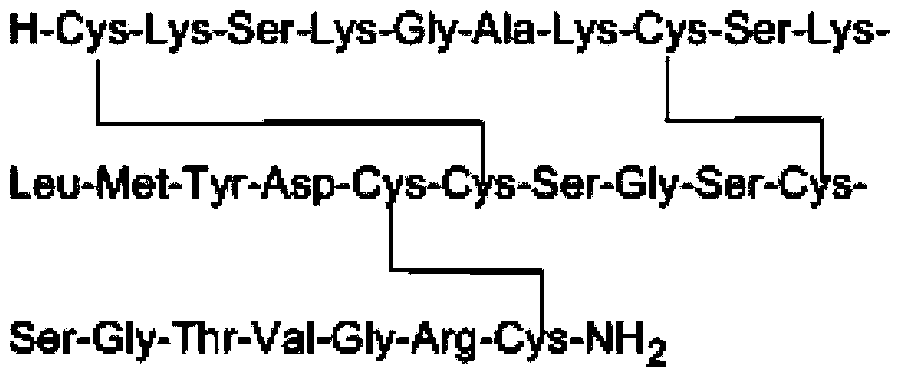Method for synthesizing leconotide
A technology of leconotide and crude peptide, which is applied in the field of drug synthesis, can solve the problems of increasing removal reaction process steps, increasing production costs, and inconvenient production processes, so as to reduce synthesis costs, shorten time, and avoid heavy metal residues Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of Fmoc-Cys(Trt)-Rink Amide Resin resin
[0038] Weigh 224g of dry Rink Amide Resin resin (the degree of substitution is 0.6mmol / g) and add it to the solid-phase reaction column. First, wash the resin twice with DMF, and swell the resin with 2 to 3 times the volume of the resin bed layer with DCM for 1 hour, and then use 20 % volume of hexahydropyridine / DMF to remove Fmoc twice, react for 5 minutes and 7 minutes respectively, wash with DMF 6 times, and wait for feeding.
[0039]Under the condition of cooling in an ice bath, dissolve 78.7g (135mmol) Fmoc-Cys(Trt)-OH, 18.3g HOAt, and 51.1g HATU in a mixed solvent of DMF and DCM. After the amino acid is dissolved, continue to keep the ice bath , slowly add TMP35.4mL, activate for 5min. Then the activated Fmoc-Cys(Trt)-OH was added to the reaction column and reacted for 2 hours. After the reaction, the resin was washed 4 times with DMF, and then the blocking solution composed of 108mL pyridine...
Embodiment 2
[0040] Embodiment 2: the preparation of Fmoc-Cys(Trt)-Rink Amide MBHA Resin resin
[0041] Weigh 243g of dry Rink Amide-MBHA Resin resin (the degree of substitution is 0.5mmol / g) and add it to the solid-phase reaction column. First, wash the resin twice with DMF, and swell the resin with 2 to 3 times the volume of the resin bed layer with DCM for 1 hour, and then Use 20% volume of hexahydropyridine / DMF to remove Fmoc twice, react for 5 minutes and 7 minutes respectively, wash with DMF 6 times, and wait for feeding.
[0042] Under the condition of cooling in an ice bath, dissolve 64.1g (110mmol) Fmoc-Cys(Trt)-OH, 16.6g HOAt, and 46.2g HATU in a mixed solvent of DMF and DCM. After the amino acid is dissolved, continue to keep the ice bath , slowly add TMP29.0mL, activate for 5min. Then the activated Fmoc-Cys(Trt)-OH was added to the reaction column and reacted for 2 hours. After the reaction, the resin was washed 4 times with DMF, and then the blocking solution composed of 97mL...
Embodiment 3
[0043] Embodiment 3: the preparation of Fmoc-Cys(Trt)-Rink Amide AM Resin resin
[0044] Weigh 215g of dry Rink Amide-AM Resin resin (the degree of substitution is 0.5mmol / g) and add it to the solid-phase reaction column. First, wash the resin twice with DMF, and swell the resin with 2 to 3 times the volume of the resin bed layer with DCM for 1 hour, and then Use 20% volume of hexahydropyridine / DMF to remove Fmoc twice, react for 5 minutes and 7 minutes respectively, wash with DMF 6 times, and wait for feeding.
[0045] Under the condition of cooling in an ice bath, dissolve 50.4g (86mmol) Fmoc-Cys(Trt)-OH, 11.7g HOAt, and 32.7g HATU in a mixed solvent of DMF and DCM. After the amino acid is dissolved, continue to keep the ice bath , slowly add TMP22.7mL, activate for 5min. Then the activated Fmoc-Cys(Trt)-OH was added to the reaction column and reacted for 2 hours. After the reaction, the resin was washed 4 times with DMF, and then 69mL of pyridine, 168ml of acetic anhydride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com