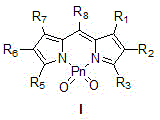
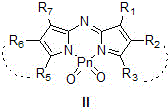
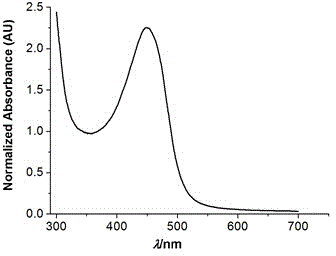
Water-soluble PnO2-PODIPY/PnO2-azaPODIPY fluorescent dye and preparation method thereof
A fluorescent dye, water-soluble technology, used in nitro/nitroso dyes, azo dyes, organic dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of Fluorescent Dye I-1
[0076]
[0077] (1) Synthesis of intermediate compound III-1.
[0078] Under argon protection, p-nitrophenylaldehyde (300 mg, 0.19 mmol) and 2,4-dimethylpyrrole (0.51 ml, 0.49 mmol) were dissolved in dichloromethane (20 ml), and a drop of trifluoro Acetic acid, stirred at room temperature, reacted for 12 h. The completion of the reaction was detected by TLC, DDQ (900 mg) was added and stirred for 30 minutes. The reaction solution was quenched with ice. It was extracted with dichloromethane, distilled under reduced pressure, and the residue was purified with a silica gel column. The developing solvent was dichloromethane / n-hexane to obtain a yellow solid III-1 (183.2 mg, 30 %).
[0079] (2) Synthesis of PO2-PODIPY dye I-1.
[0080] Under an open system, the obtained solid III-1 (64.2 mg) was dissolved in dichloromethane (20 ml), then triethylamine (0.7 ml) was added, and reacted for 10 minutes. Then, phosphorus oxychloride (1...
Embodiment 2
[0082] Preparation of fluorescent dye IIa-1
[0083]
[0084] (1) Synthesis of intermediate compound IVa-1
[0085] In an open system, add 2,4-diphenylpyrrole (43.8 mg, 0.2 mmol), glacial acetic acid (1 ml), acetic anhydride (0.4 ml), add sodium nitrite (6.9 mg, 0.1 mmol) under ice cooling, React at room temperature for 0.5 h, transfer to 80 °C for 0.5 h, and the reaction ends. The reaction was quenched by adding ice water, a solid would precipitate out, and the solid was filtered out. The filter cake was dissolved in dichloromethane, passed through the column with dichloromethane as the eluent and alumina as the stationary phase, and the solvent was spin-dried to obtain blue-purple solid IVa-1 (35.9 mg, 80%). 1 H NMR (400 MHz, CDCl 3 ) 8.04-8.08 (m, 4H), 7.94-7.97 (m, 4H), 7.33-7.57 (m, 12H), 7.21 (s, 2H) (NH not observed). 13 C NMR (CDCl3) 155.1, 149.6, 142.7, 133.7, 132.2, 130.1, 129.2, 129.1, 128.3, 128.0, 126.6, 114.9.
[0086] (2) Synthesis of PO2-azaPODIPY d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com