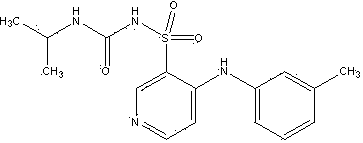
Torasemide compound
A technology for torsemide and compounds, applied in the field of medicine, can solve the problems of unreproducible content and crystal form, difficulty in scaling up the pilot scale, low purity of torsemide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
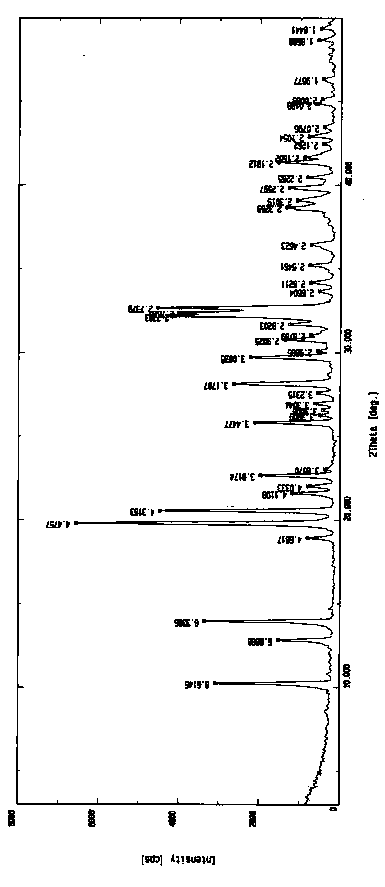
[0046] In a 50L reactor, add 3 kg of torasemide (purity 97.3%, HPLC) and 30.3 L of water-acetone-ammonia = 9:0.8:1.1 mixture, heat to 88°C-91°C, add 150 gram of activated carbon, insulated and stirred for 30 minutes, filtered while hot, naturally cooled to room temperature under filtrate stirring, and then incubated for 5.5 hours, crystallization was precipitated, filtered, and vacuum-dried at room temperature to obtain 2.81 kilograms of torasemide crystals. Melting point: 148.5°C-149.6°C, purity 99.95%, single impurity 0.04%, MS: 349.13 (M+H) solvent residue detection meets the requirements.
[0047] Elemental Analysis Results:
[0048]Measured value (calculated value), C: 55.25 (55.16), H: 5.68 (5.79), N: 16.02 (16.08), S9.18 (9.20).
[0049] The X-ray diffraction pattern of the crystal is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° .
Embodiment 2
[0051] The injection containing the torasemide crystal of the present invention is prepared by using standard and conventional techniques, the specification: 10 mg / bottle.
[0052] Weigh 5 grams of sodium chloride, add 1800 milliliters of water for injection, and stir to dissolve it. In addition, 10 grams of torasemide crystals of the present invention are weighed, added to the above solution, adjusted to pH 5.5-6.5 with dilute hydrochloric acid under stirring, 1 gram of activated carbon is added, stirred for 30 minutes, filtered and decarburized to make the liquid clear, and added to the injection Water to 2000 ml, stir evenly, filter with microporous membrane, fill 2ml into a vial after the content is qualified, half-stoppered, freeze-dried in a freezer, fully stoppered, and take out the product after the vacuum is released and tie an aluminum cap. Inspect and pack.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com