A Method for Predicting the Skin Permeability Coefficient of Organic Chemicals
A technology of organic chemicals and skin penetration, applied in the direction of electrical digital data processing, special data processing applications, instruments, etc., can solve problems such as unfavorable model application and mechanism interpretation, inability to extract prediction rules, poor comprehensibility, etc., to facilitate analysis and practical application, wide coverage of activity, strong predictive ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: The given chemical substance 6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine (SMILES: CCNclnc(Cl)nc(NC(C )C)n1), predict its skin permeability coefficient.
[0037] First, according to the molecular structure of the chemical substance, seven descriptors BEHm8, GGI2, RDF030v, Mor17v, G2s, H5m, and RTu+ were calculated using Dragon software; they were 1.893, 0.667, 0.959, -0.171, 0.208, 0.034, and 0.151, respectively. Hat is 0.213, within the scope of the model application domain, this model can be used for the skin permeability coefficient of 6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine For prediction, the descriptor values are substituted into the built model as follows:
[0038] Log Fl=–0.323+1.893*(-0.51007)+0.667*(-0.31582)+0.959*(-0.06401)
[0039] -0.171*(-2.17293)+0.208*(-0.44581)+0.034*1.58672+0.151*2.54638=-1.83
[0040] Then 6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine is predicted to be -1.83, and the experim...
Embodiment 2
[0042] Prediction of skin permeability coefficient given 1,1,1-trichloroethane (SMILES: CC(Cl)(Cl)Cl). First, according to the molecular structure of the chemical substance, use Dragon software to calculate 7 kinds of descriptors BEHm8, GGI2, RDF030v, Mor17v, G2s, H5m, RTu+, which are 0, 0, 1.253, -0.044, 0.536, 0, 0.219, and Hat is 0.038 , within the scope of the model application domain, this model can be used to predict the skin permeability coefficient of 1,1,1-trichloroethane, and the descriptor values are substituted into the built model as follows:
[0043] Log Fl=–0.323+0*(-0.51007)+0*(-0.31582)+1.253*(-0.06401)-0.044*(-2.17293)+
[0044] 0.536*(-0.44581)+0*1.58672+0.219*2.54638=0.23
[0045] Then 1,1,1-trichloroethane is predicted to be 0.23, and the experimental value is 0.21, which is close to the experimental results.
Embodiment 3
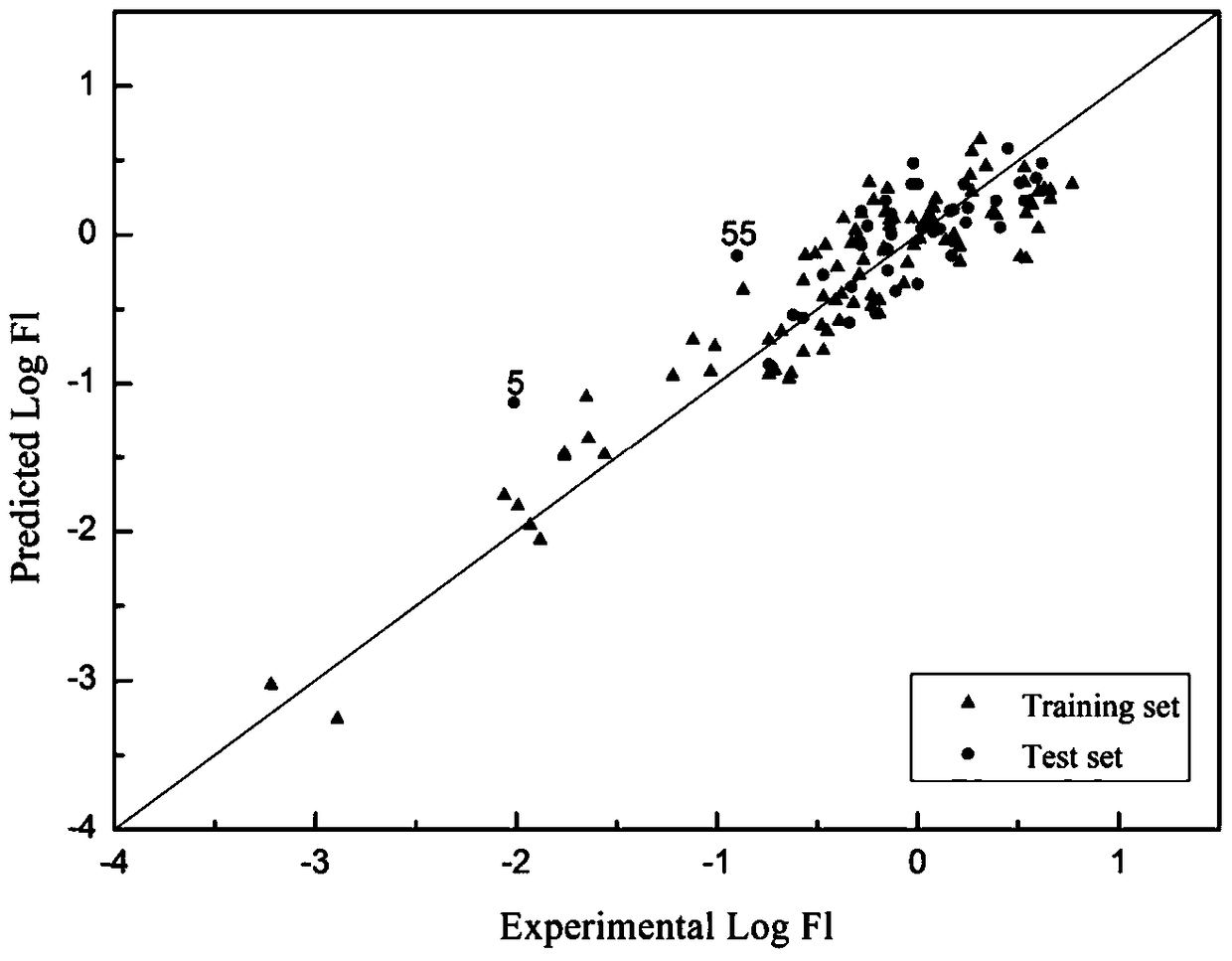
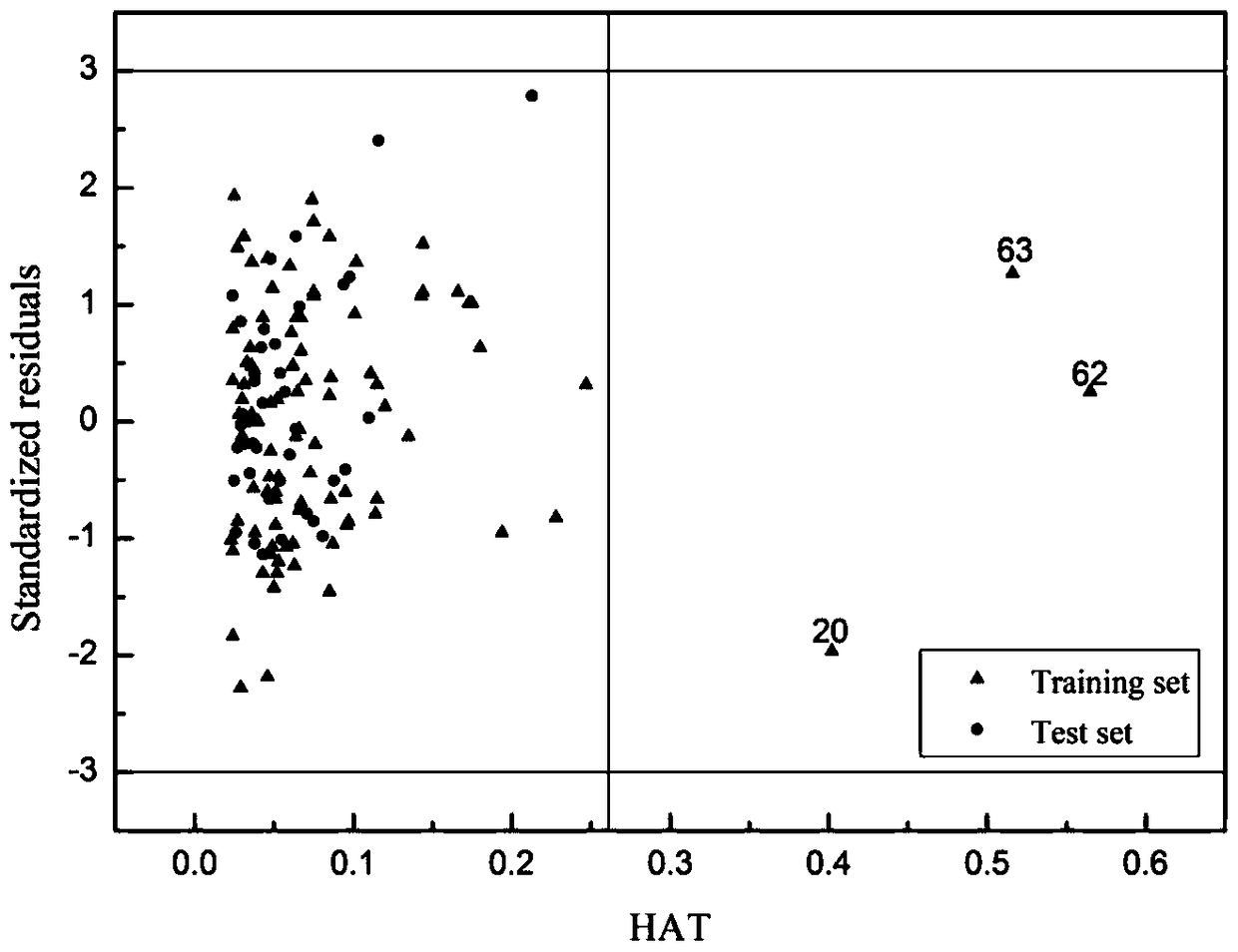
[0047] Given O,O-dimethyl-O-(2,4,5-trichlorophenyl)phosphorothioate (SMILES: COP(=S)(OC)Oc1cc(c(cc1Cl)Cl)Cl) prediction its skin permeability coefficient. First, according to the molecular structure of the chemical substance, use the Dragon software to calculate 7 kinds of descriptors BEHm8, GGI2, RDF030v, Mor17v, G2s, H5m, RTu+, respectively 2.335, 2.667, 2.392, 0.078, 0.222, 1.087, 0.185, Hat is 0.565, Not within the scope of the model's application domain. Since the scope of application of the model is analyzed and displayed with leverage and Williams diagrams. The abscissa of the Williams diagram is the leverage value (hat), and the ordinate is the standard residual (σ), from which we can see the X exception point and the Y exception point. There are no Y exception points in the Williams diagram training set and verification set data of the model, and there are no X exception points in the verification set data. Compound 20, compound 62 and compound 63 in the training s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com