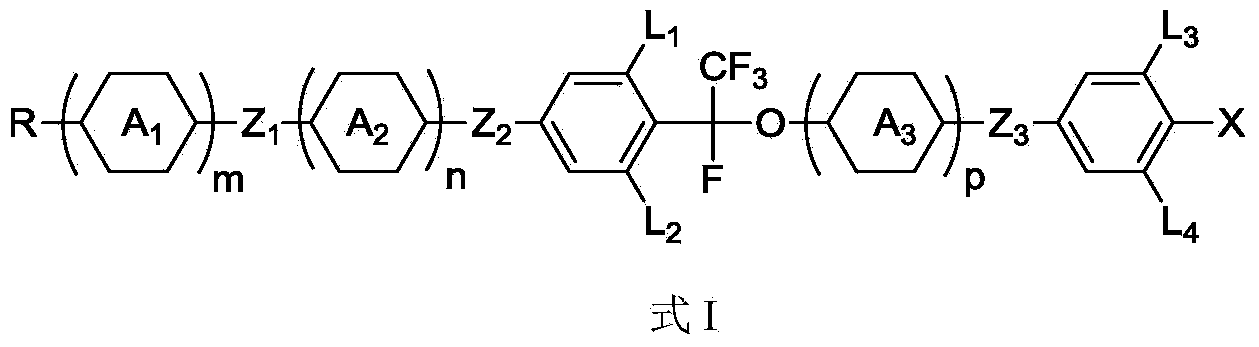
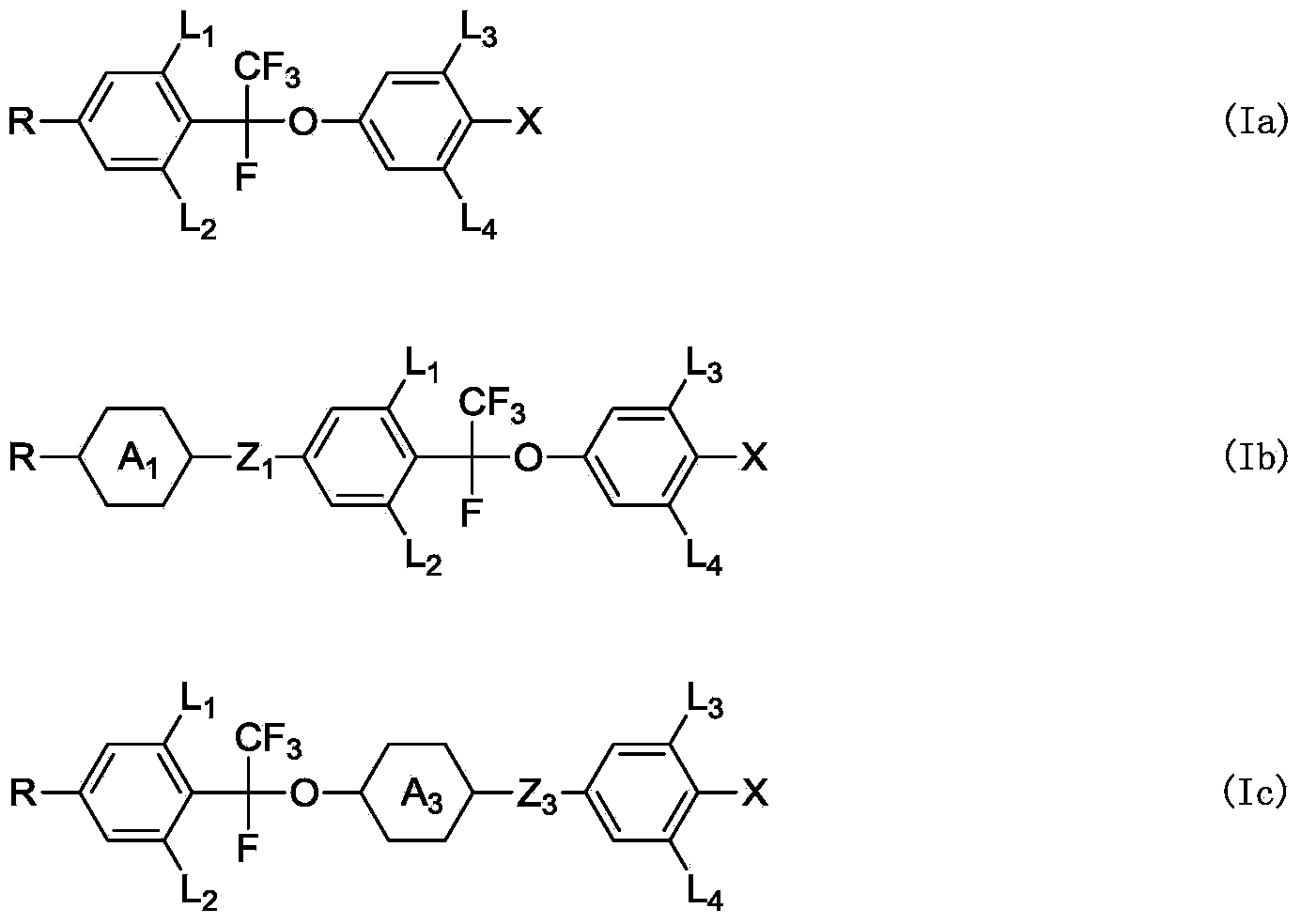
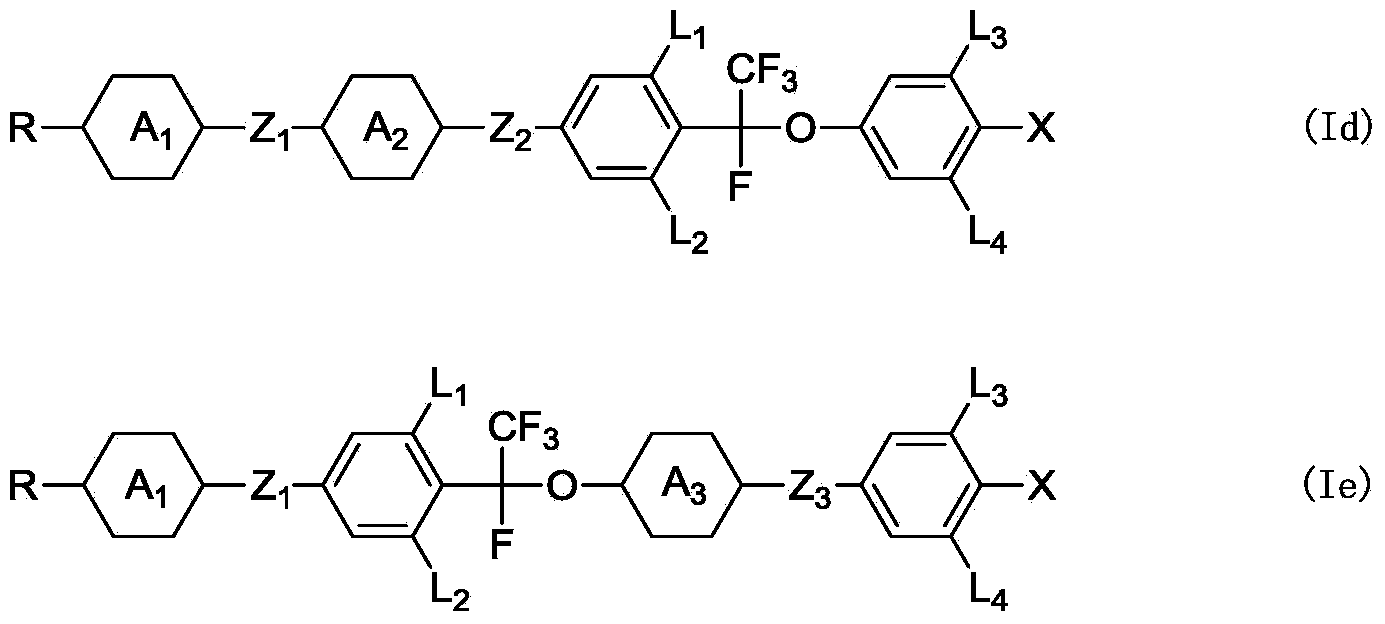
Liquid crystal compound, preparation method and applications thereof
A compound and reaction technology, applied in chemical instruments and methods, ether preparation, liquid crystal materials, etc., can solve the problems of no reports, the reduction of the clearing point of liquid crystal, and the space that restricts the improvement of the response speed of liquid crystal mixtures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Compound I514 shown in embodiment 1, formula I (method 1)
[0072]
[0073] step 1
[0074]
[0075] 25g (69.8mmol) (I514-0), 14.8g (139.6mmol) of anhydrous sodium carbonate, 1L of 1,4-dioxane and 50ml of water were added to a 2L pressure-resistant reactor, and 0.57g (0.698mmol) was added PdCl 2 (dppf) catalyst, filled with nitrogen to replace the air, placed in a low-temperature tank and cooled with liquid nitrogen, when the temperature dropped to -80 °C, 32.5 g (0.28 mol) of gas chlorotrifluoroethylene was introduced, the reaction vessel was sealed, and the temperature was raised to 100 °C and stirred After reacting for 2 hours, cool to room temperature, add 200ml ethyl acetate and 50ml saturated aqueous ammonium chloride solution, separate the layers, extract the aqueous phase twice with 100ml ethyl acetate, and combine the organic layers.
[0076] The solvent was evaporated under reduced pressure, dissolved in petroleum ether, passed through a silica gel col...
Embodiment 2
[0094] Embodiment 2, compound I317 shown in preparation formula I (method 2)
[0095]
[0096] step 1
[0097]
[0098] In a 2L three-neck flask, under a nitrogen atmosphere, add 141.5g (0.5mol) of (I317-0), 400ml of tetrahydrofuran, place it in a low-temperature tank and cool it with liquid nitrogen, and when it drops to -80°C, slowly add 220ml (2.5 M, 0.55mol) n-butyllithium hexane solution, added in about 2 hours, continued to stir for 1 hour, and added 156g (0.6mol) of 1,1-dibromotetrafluoroethane dropwise to the solution under stirring. In 150ml of tetrahydrofuran solution, add for about 2.5 hours, the reaction is exothermic, and then stir for another 2 hours after the addition.
[0099] Add 300ml of dichloromethane for dilution, add 300ml of 2N dilute hydrochloric acid aqueous solution with stirring, and stir for 10 minutes. Separation, the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, the solvent was evaporated u...
Embodiment 3
[0109] Embodiment 3, compound I499 shown in preparation formula I (method 2)
[0110]
[0111] step 1
[0112]
[0113] Add 32g (0.1mol) (I499-0) and 250ml tetrahydrofuran to a 1L three-necked flask, replace the air with nitrogen, place it in a low-temperature tank and cool it with liquid nitrogen, and when it drops to -78°C, add 44ml of 2.5M butyllithium solution dropwise ( 0.11mol), add in about 30 minutes, keep warm and stir for 1 hour, add 39g (0.15mol) of 1,1-dibromotetrafluoroethane dropwise into the reaction flask, and stir for 2 hours at -78°C. Add 200ml of dichloromethane for dilution, add 200ml of 2N dilute hydrochloric acid aqueous solution with stirring, and stir for 10 minutes. Separation, the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, the solvent was evaporated under reduced pressure, dissolved in petroleum ether, passed through a silica gel column, washed with petroleum ether, and the solvent was evapor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com