Series one-pot synthesis method of lithium oxalyldifluroborate
A technology of lithium difluoroborate oxalate and its synthesis method, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., and can solve the problem of silyl oxalate synthesis route that is not suitable for rapid production requirements Complicated and complicated problems, to achieve the effect of fast response and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
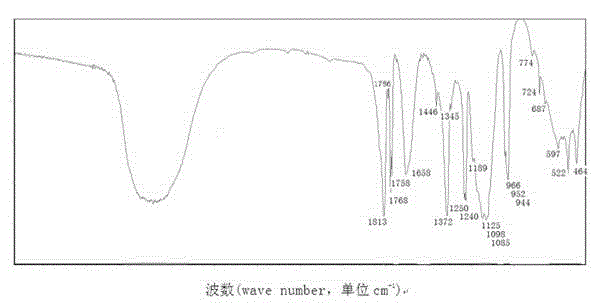
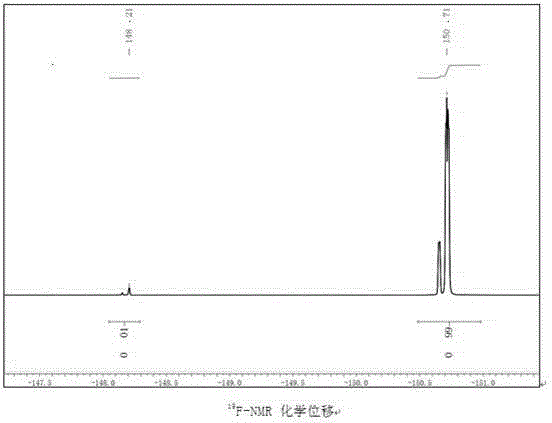
Image
Examples
Embodiment 1
[0031] Lithium oxalate difluoroborate was prepared from boron trifluoride ether solution, lithium oxalate, trimethylmethoxysilane and oxalic acid. In a reaction flask equipped with a magnetic stirrer, 101.9 g of lithium oxalate was added, 500 mL of acetonitrile was added, stirring was started, and 288 g of boron trifluoride-diethyl ether solution was slowly added dropwise therein. Continue to stir after the dropwise addition is complete. After all the solid materials are dissolved, add 90g of oxalic acid and slowly dropwise add 208.4g of trimethylmethoxysilane. The reaction pressure is 0.1Mpa, and the reaction is carried out at 20°C for 10 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally. The solvent and by-products were distilled off under reduced pressure. The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of 60-80° C. for 48...
Embodiment 2
[0034] Lithium oxalate difluoroborate was prepared from boron trifluoride ether solution, lithium fluoride, trimethylethoxysilane and oxalic acid.
[0035] In a reactor equipped with a heating and magnetic stirring device, 25.9 g of lithium fluoride and 500 mL of dimethyl carbonate were added, stirring was started, and 144.2 g of boron trifluoride-diethyl ether solution was slowly added dropwise therein. After the dropwise addition is completed, continue to stir until all solid materials are dissolved. Add 90g of oxalic acid, slowly add 236.5g of trimethylethoxysilane at room temperature, the reaction pressure is 0.2Mpa, heat to 80°C, and react for 2 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally.
[0036] The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of 60-80° C. for 48 hours. Finally, 115 g of white powd...
Embodiment 3
[0039] Lithium oxalate difluoroborate was prepared from boron trifluoride acetonitrile solution, lithium fluoride, boron trichloride and oxalic acid.
[0040] In a reactor equipped with a heating and magnetic stirring device, 77.7 g of lithium fluoride and 1000 mL of diethyl carbonate were added, stirring was started, and 144.2 g of boron trifluoride-diethyl ether solution was slowly added dropwise therein. After the dropwise addition, continue to stir until all solid materials are dissolved, slowly add 235.4g of boron trichloride and 270g of oxalic acid at room temperature, the reaction pressure is 0.15Mpa, heat to 60°C, and react for 4 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally.
[0041] The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of 60-80° C. for 48 hours. Finally, 388.2 g of white powdery solids w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com